Be sure to read our Editor’s Choice articles as chosen by Associate Editors Prof. Jonathan Steed & Prof. Jonathan Sessler!
All articles are free-to-access until 31st August and can be found in our online Editor’s Choice web-collection!


“Planar rings in nano-Saturns and related complexes” by Steven M. Bachrach, as chosen by Jonathan Steed:
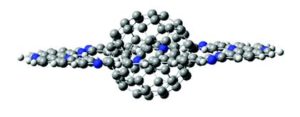
“This paper lays down the gauntlet to synthetic chemists! The image of a nano-Saturn is immediately eye-catching and scales up molecular host guest chemistry to the multi-nanometre scale. This creative theoretical paper establishes that ortho-nitrogen substitution in aryl macrocycles creates large planar or ribbon structures and then goes on to show that these discs or rings can combine with other nanostructures to construct complexes with interesting shapes. Given the huge interest generated by the mechanically interlocked structures underlying the 2016 Nobel prize in chemistry, these large-scale included systems are real food for thought and I am excited to see if they can be realised experimentally.”
“Enhancing selectivity of cation exchange with anion receptors” by Vyacheslav S. Bryantsev and Bruce A. Moyer et al., as chosen by Jonathan Sessler:
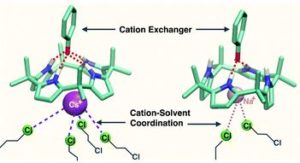
“These researchers have shown that by using a classic anion binding agent, namely a calix[4]pyrrole, it is possible to modulate the inherent selectivity of liquid-liquid cation extractants. Most current extraction-based separations rely on the use of lipophilic anions as the extractants. These anions, typically the conjugate bases of carboxylic acids, beta-diketones, phosphoric/phosphonic/phosphinic acids, phenols, hydroxyoximes, and sulfonic acids, complex to the cation in question with a selectivity set largely by the local anion-cation coordination environment. However, in this communication the ORNL team has shown that when a calix[4]pyrrole is added to a phenolate-type cation extractant the inherent selectivity is pushed in favor of Cs+ over Na+. This bias in favor of Cs+, which stands in contrast to what would normally be expected, is rationalized in terms of the formation of a highly specific tertiary supramolecular complex involving the calix[4]pyrrole, the anionic phenolate, and the Cs+ cation. Such an organized ternary complex is disfavored in the case of Na+. This work is particularly appealing to me as an Associate Editor for its combination of novelty, insightfulness, and scholarly rigor. It is also attractive to me personally because it demonstrates a new utility for one of my favorite old-but-new molecules, namely calix[4]pyrrole.”
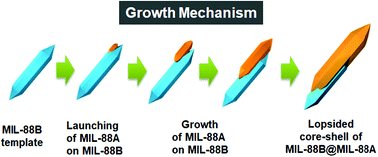
Bonus article: “p-Phosphonic acid calix[8]arene mediated synthesis of ultra-large, ultra-thin, single-crystal gold nanoplatelets” by Chuan Zhao, Colin L. Raston & Xianjue Chen et al., as chosen by Jonathan Steed:
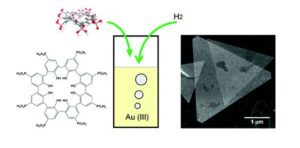 “This work reports a very simple system that gives glorious gold nanoplatelets with significant surface area but a thickness of around 6nm. Creating 2D nanocrystals is very challenging and involves highly kinetic conditions. In this case the simple reduction of soluble gold(III) in the presence of a phosphonated acid calix[8]arene macrocycle gives rise to these very well-defined and very unusual morphologies. In this case the role of the calixarene seems to be to attach to the Au(111) surfaces, impeding their growth in one direction and allowing growth in the other to form single-crystal platelets. We are still just scratching the surface of what unusual nanoscale morphologies can do to alter the properties of a material but the present gold nanowafers already show promise as oxygen sensors.”
“This work reports a very simple system that gives glorious gold nanoplatelets with significant surface area but a thickness of around 6nm. Creating 2D nanocrystals is very challenging and involves highly kinetic conditions. In this case the simple reduction of soluble gold(III) in the presence of a phosphonated acid calix[8]arene macrocycle gives rise to these very well-defined and very unusual morphologies. In this case the role of the calixarene seems to be to attach to the Au(111) surfaces, impeding their growth in one direction and allowing growth in the other to form single-crystal platelets. We are still just scratching the surface of what unusual nanoscale morphologies can do to alter the properties of a material but the present gold nanowafers already show promise as oxygen sensors.”
Find our full Editor’s Choice collection online!
Keep up-to-date with our latest journal news on Twitter @ChemCommun!
Learn more about ChemComm online! Submit your latest high impact research here!