This month sees the following articles in ChemComm that are in the top ten most accessed:-
Porous metal-organic frameworks as platforms for functional applications
Hai-Long Jiang and Qiang Xu
Chem. Commun., 2011, 47, 3351-3370, DOI: 10.1039/C0CC05419D, Feature Article
Surface modification of polyoxometalate host-guest supramolecular architectures: from metal-organic pseudorotaxane framework to molecular box
Xiaofei Kuang, Xiao-Yuan Wu, Jian Zhang and Can-Zhong Lu
Chem. Commun., 2011, 47, 4150-4152, DOI: 10.1039/C0CC05855F, Communication
Homogeneous catalysis using iron complexes: recent developments in selective reductions
Kathrin Junge, Kristin Schröder and Matthias Beller
Chem. Commun., 2011, 47, 4849-4859, DOI: 10.1039/C0CC05733A, Highlight
How easy are the syntheses of allenes?
Shichao Yu and Shengming Ma
Chem. Commun., 2011, 47, 5384-5418, DOI: 10.1039/C0CC05640E, Feature Article
Fe3O4 nanostructures: synthesis, growth mechanism, properties and applications
Ce Yang, Jiajia Wu and Yanglong Hou
Chem. Commun., 2011, 47, 5130-5141, DOI: 10.1039/C0CC05862A, Feature Article
Ordered mesoporous materials as adsorbents
Zhangxiong Wu and Dongyuan Zhao
Chem. Commun., 2011, 47, 3332-3338, DOI: 10.1039/C0CC04909C, Highlight
Mn12 single-molecule magnet aggregates as magnetic resonance imaging contrast agents
Yinglin Wang, Wen Li, Shengyan Zhou, Daliang Kong, Haishan Yang and Lixin Wu
Chem. Commun., 2011, 47, 3541-3543, DOI: 10.1039/C0CC03758C, Communication
A Covalent Organic Framework with 4 nm open pores
Mirjam Dogru, Andreas Sonnauer, Andrei Gavryushin, Paul Knochel and Thomas Bein
Chem. Commun., 2011, 47, 1707-1709, DOI: 10.1039/C0CC03792C, Communication
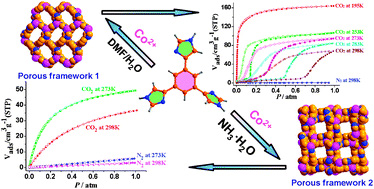
Selective CO2 adsorption in a flexible non-interpenetrated metal-organic framework
Tae Kyung Kim and Myunghyun Paik Suh
Chem. Commun., 2011, 47, 4258-4260, DOI: 10.1039/C0CC05199C, Communication
A colorimetric and fluorescent chemosensor for the detection of an explosive-2,4,6-trinitrophenol (TNP)
Yu Peng, Ai-Jiang Zhang, Ming Dong and Ya-Wen Wang
Chem. Commun., 2011, 47, 4505-4507, DOI: 10.1039/C1CC10400D, Communication
Why not take a look at the articles today and blog your thoughts and comments below.
Fancy submitting an article to ChemComm? Then why not submit to us today or alternatively contact us with your suggestions.