A new type of bismuth-based electrode material for sodium-ion batteries has been synthesized. This electrode consists of bismuth metal nanoflakes seamlessly integrated onto nickel foams. The electrode contains no polymer binders, a crucial component required to retain the structural integrity of most battery electrodes. This binder-free feature improves the amount of charge being stored (i.e. capacity) at fast charging rates.
Sodium-ion batteries are attracting worldwide research efforts as electric energy storage devices, in addition to the prevalent lithium-ion batteries, due to the abundance of sodium. Similar to the preparation of other battery electrodes, fabricating sodium-ion battery electrodes generally requires binders, e.g. polyvinylidene fluoride (PVDF), to hold powdered electrode materials together and glue them to metal supporting substrates. However, the electrically insulating nature of the binders impedes fast electron transport between electrode materials and supporting substrates, consequently degrading the capacity of the batteries at fast charging rates.
Now in ChemComm, researchers from Nankai University & the Collaborative Innovation Center of Chemical Science and Engineering in China demonstrate a bismuth-based electrode material that does not involve a binder. This characteristic is realized by the in-situ growth of bismuth nanoflakes onto nickel foams through a solution-based replacement reaction (Figure 1). Because the nanoflakes grow directly from the nickel foam surface and firmly anchor onto nickel (Figure 2a), the resultant Bi/Ni composite can be directly used as an electrode. Specifically, the bismuth nanoflakes and nickel foam serve as the active material and supporting substrate, respectively.
The Bi/Ni composite exhibited excellent electrochemical performance. It achieved a high capacity of 377.1 mAh/g at a current density of 20 mA/g. Significantly, when the current density increased 100-fold, its capacity could still retain 206.4 mAh/g, which is more than half of the capacity obtained at 20 mA/g (Figure 2b). This outstanding capacity retention is a benefit of the binder-free characteristic that reduces the resistance of electron transport.
The authors then elucidated the working mechanism of the bismuth nanoflakes by in-situ Raman spectroscopy. They concluded that a two-step alloying process was responsible for the charge storage activity.
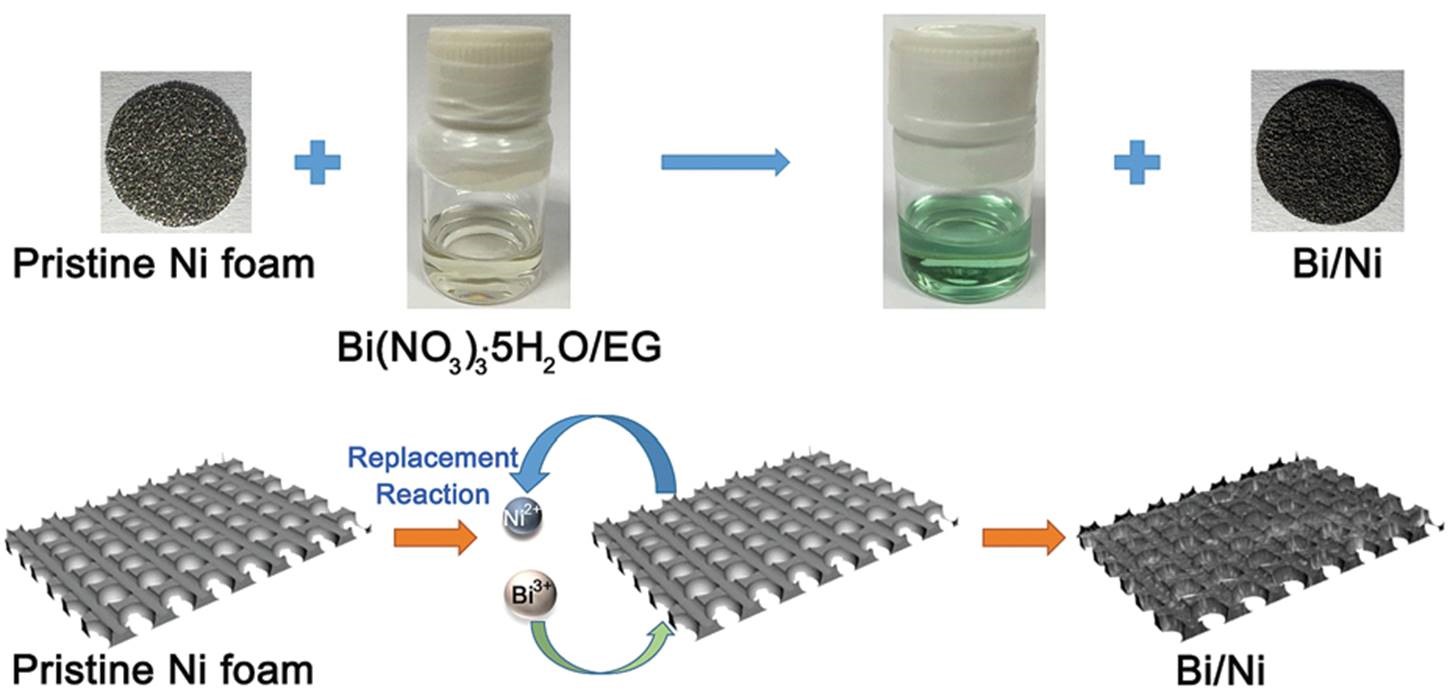
Figure 1. A schematic illustration showing the synthetic process of the binder-free Bi/Ni electrode. By inserting a piece of nickel foam into an ethylene glycol (EG) solution containing bismuth(III) nitrate, Bi3+ can replace Ni metal, be reduced to Bi metal and deposit on the Ni metal surface.

Figure 2. (a) A scanning electron microscopy image of the bismuth nanoflakes. (b) A plot showing the capacity of the Bi/Ni electrode at different current densities.
The successful synthesis of the binder-free electrode is expected to encourage future works on the design and synthesis of integrated electrode materials to advance the performance of sodium-ion batteries.
To find out more please read:
In situ Synthesis of Bi Nanoflakes on Ni Foam for Sodium-ion Batteries
Liubin Wang, Chenchen Wang, Fujun Li, Fangyi Cheng and Jun Chen
Chem. Commun. 2017, DOI: 10.1039/c7cc08341f
About the blogger:
 Tianyu Liu obtained his Ph.D. in Physical Chemistry from University of California, Santa Cruz in United States. He is passionate about scientific communication to introduce cutting-edge research to both the general public and scientists with diverse research expertise. He is a web blog writer for Chem. Commun. and Chem. Sci. More information about him can be found at http://liutianyuresearch.weebly.com/.
Tianyu Liu obtained his Ph.D. in Physical Chemistry from University of California, Santa Cruz in United States. He is passionate about scientific communication to introduce cutting-edge research to both the general public and scientists with diverse research expertise. He is a web blog writer for Chem. Commun. and Chem. Sci. More information about him can be found at http://liutianyuresearch.weebly.com/.
Comments Off on Binder-free Integration of Bismuth Nanoflakes onto Nickel Foams for Sodium-ion Batteries














 Tianyu Liu obtained his Ph.D. in Physical Chemistry from University of California, Santa Cruz in United States. He is passionate about scientific communication to introduce cutting-edge research to both the general public and scientists with diverse research expertise. He is a web blog writer for Chem. Commun. and Chem. Sci. More information about him can be found at
Tianyu Liu obtained his Ph.D. in Physical Chemistry from University of California, Santa Cruz in United States. He is passionate about scientific communication to introduce cutting-edge research to both the general public and scientists with diverse research expertise. He is a web blog writer for Chem. Commun. and Chem. Sci. More information about him can be found at