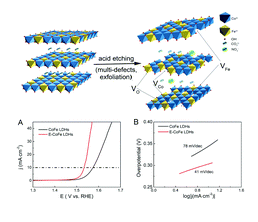
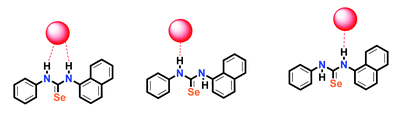
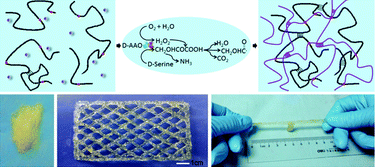
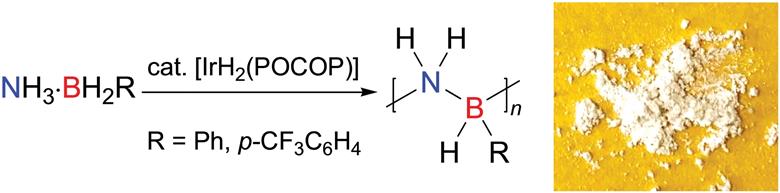
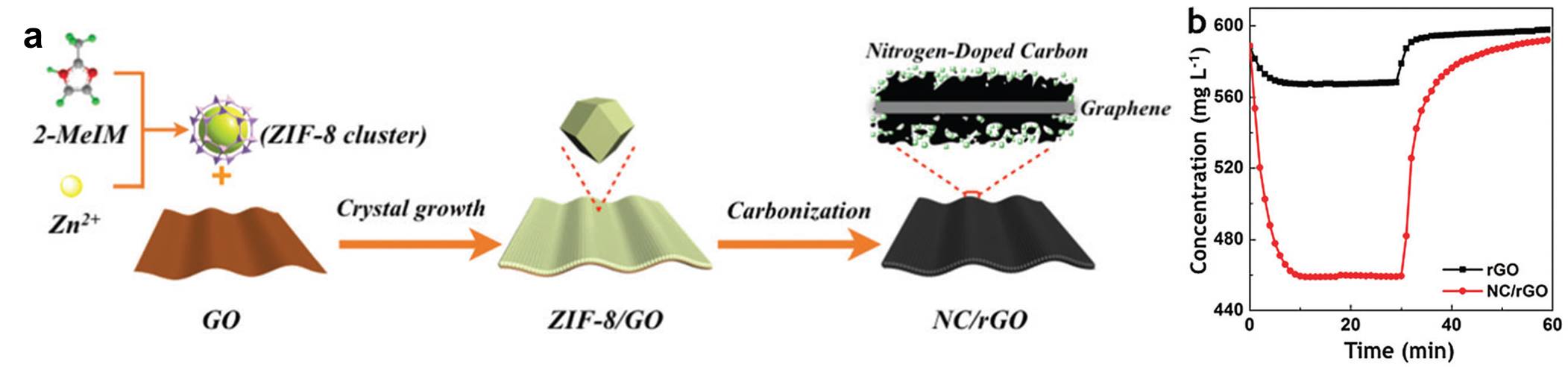
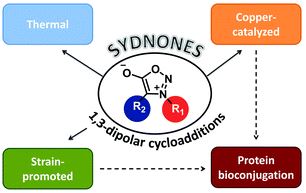
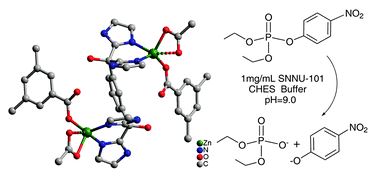
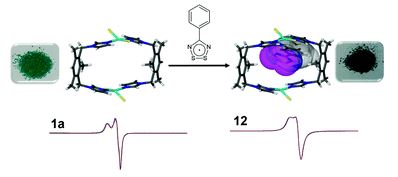
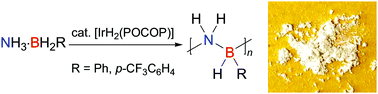The International Committee of the International Symposium on Macrocyclic and Supramolecular Chemistry is pleased to invite nominations for the Cram Lehn Pedersen Prize for young supramolecular chemists.
The Cram Lehn Pedersen Prize, named in honour of the winners of the 1987 Nobel Prize in Chemistry, recognises significant original and independent work in supramolecular chemistry.
Previous winners include Tom F. A. de Greef, Ivan Aprahamian, Feihe Huang, Oren Schermann, Tomoki Ogoshi, Jonathan Nitschke, and Amar Flood.
Those who are within 10 years of receiving their PhD on 31st December 2017 are eligible for the 2018 award. The winner will receive a prize of £2000 and free registration for the ISMSC meeting in Québec, Canada. In addition to giving a lecture at ISMSC, a short lecture tour will be organised after the meeting in consultation with the Editor of Chemical Communications, the sponsor of the award.
Nomination Details:
You may nominate yourself or someone else. Please send your CV, list of publications (divided into publications from your PhD and post-doc, and those from your independent work), and if desired, a letter of support, or these materials for someone you wish to nominate, to Prof. Roger Harrison (ISMSC Secretary) at rgharris@chem.byu.edu by 31st December 2017.












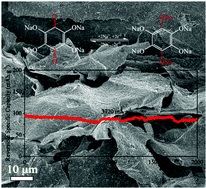
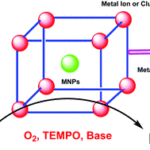
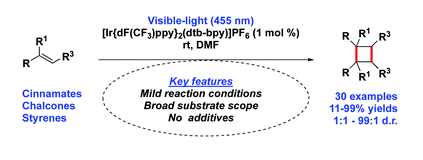
 Tianyu Liu obtained his Ph.D. in Physical Chemistry from University of California, Santa Cruz in United States. He is passionate about scientific communication to introduce cutting-edge research to both the general public and scientists with diverse research expertise. He is an online blog writer for Chem. Commun. and Chem. Sci. More information about him can be found at
Tianyu Liu obtained his Ph.D. in Physical Chemistry from University of California, Santa Cruz in United States. He is passionate about scientific communication to introduce cutting-edge research to both the general public and scientists with diverse research expertise. He is an online blog writer for Chem. Commun. and Chem. Sci. More information about him can be found at