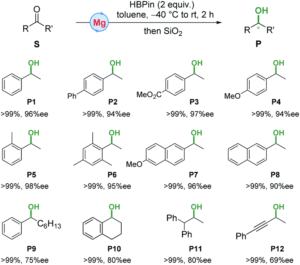
Before I get into the research, I just have to say that I think ionic liquids (ILs) are just cool. An ionic compound that’s a liquid? Mind blown. Not only are they interesting, but those unique properties make them attractive for industrial use. One class of ionic liquids generating research focus is halometallate ionic liquids (HILs), generated by reacting a metal halide and an organic halide salt. In particular, chloro-alluminate ILs have been some of the most promising for applications in industrial acid catalysis and are composed of anionic aluminate species. However, recent work has found mixtures of aluminum chloride and N/O/S donors produce a liquid Lewis acidic compounds, referred to as liquid coordination complexes (LCCs). These LCCs are potential replacements for HILs, as they’re typically easier and cheaper to prepare. Further studies have found ionic species by 27Al NMR, drawing parallels between LCCs and HILs. By combining AlCl3 and polar organic solvents, researchers in the US screened for novel LCC or HIL reactivity in catalysis.
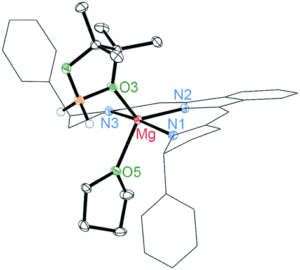
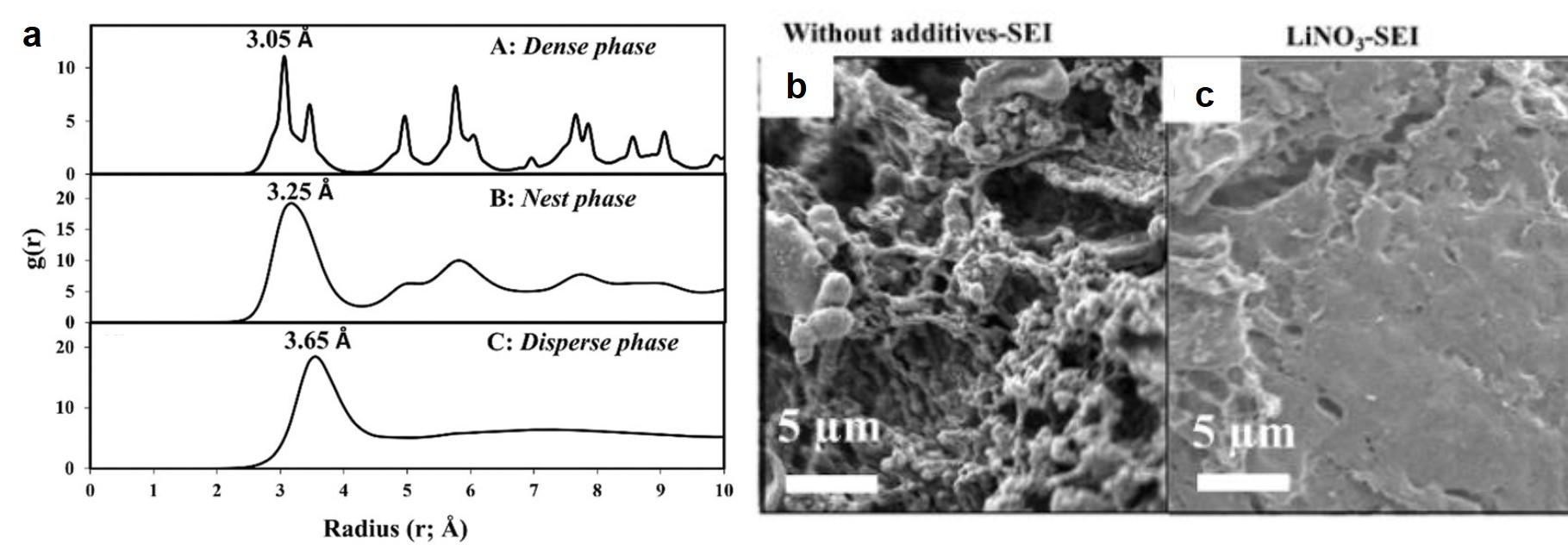
This straightforward approach allowed researchers to easily tune the ratio of AlCl3 and solvent to find mixtures with desired properties. They chose the nitrogen donor 1-methylimidazole, N-Mim, and an oxygen donor N-methyl-2-pyrrolidone (O-NMP) as their solvents for testing given their wide availability and relevance to organic chemical reactivity. The selected solvent and AlCl3 were mixed at room temperature and found in all cases to form heterogenous mixtures. When heated to 100 oC mixtures with molar fractions of AlCl3 between 0.33 and 0.6 formed viscous liquids, many of which became solids at room temperature. These compounds were then crystalized for x-ray crystallographic analysis, where their structures confirmed the association of the aluminum with the nitrogen (Figure 1) or oxygen.
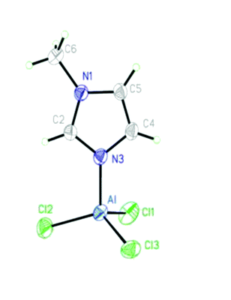
Figure 1. 50% probability ellipsoid plot of AlCl3(N-Mim), showing coordination between the aluminum and nitrogen
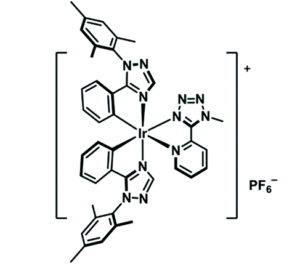
In the N-Mim system, 27Al NMR showed the formation of a single aluminum-containing species at AlCl3 molar fractions of 0.5 and below, with exchange occurring when higher concentrations of N-Mim are present. The O-NMP system proved more challenging to characterize crystallographically, potentially due to the formation of larger oligomeric complexes in the solid phase and increased disorder in the O-NMP ligand. However, 27Al NMR proved insightful and showed the presence of multiple aluminum-containing species, including several different stoichiometries of aluminum-solvent adducts.
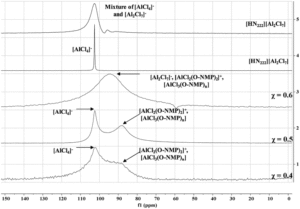
Figure 2. Aluminum NMR spectra of aluminum/O-NMP complexes showing speciation over a range of different stoichiometries.
When the two systems were side-by-side compared for Lewis acidity and catalytic activity for the alkylization of benzene, the clear winner was the O-NMP system. The O-NMP-AlCl3 complex with an aluminum molar fraction of 0.6 was both the most Lewis acidic, determined by an acetonitrile IR probe, and the most catalytically active. It gave full conversion with a selectivity of almost 80%, while the N-Mim complex with the same mole fractions produced only a 32% conversion with no significant increase in selectivity. Complexes with less aluminum showed no signs of catalytic activity and were less Lewis acidic. The high activity of the AlCl3/O-NMP system can be explained by its possession of both a highly Lewis acidic cation [AlCl2(O-NMP)2]+ and a highly Lewis acidic anion [Al2Cl7]–, whose presence was identified in the NMR experiments. This work demonstrated a straightforward method to synthesize LCC-based catalysts with high activity, while providing some general guidance on the suitability of O-donor ligands for further study.
To find out more, please read:
Rajkumar Kore, Steven P. Kelley, Anand D. Sawant, Manish Kumar Mishra and Robin D. Rogers
Chem. Commun., 2020, Advance Article
About the blogger:
 Dr. Beth Mundy is a recent PhD in chemistry from the Cossairt lab at the University of Washington in Seattle, Washington. Her research focused on developing new and better ways to synthesize nanomaterials for energy applications. She is often spotted knitting in seminars or with her nose in a good book. You can find her on Twitter at @BethMundySci.
Dr. Beth Mundy is a recent PhD in chemistry from the Cossairt lab at the University of Washington in Seattle, Washington. Her research focused on developing new and better ways to synthesize nanomaterials for energy applications. She is often spotted knitting in seminars or with her nose in a good book. You can find her on Twitter at @BethMundySci.













 Tianyu Liu obtained his Ph.D. (2017) in Chemistry from the University of California, Santa Cruz, in the United States. He is passionate about the communication of scientific endeavors to both the general public and other scientists with diverse research expertise to introduce cutting-edge research to broad audiences. He is a blog writer for Chem. Comm. and Chem. Sci. More information about him can be found at
Tianyu Liu obtained his Ph.D. (2017) in Chemistry from the University of California, Santa Cruz, in the United States. He is passionate about the communication of scientific endeavors to both the general public and other scientists with diverse research expertise to introduce cutting-edge research to broad audiences. He is a blog writer for Chem. Comm. and Chem. Sci. More information about him can be found at