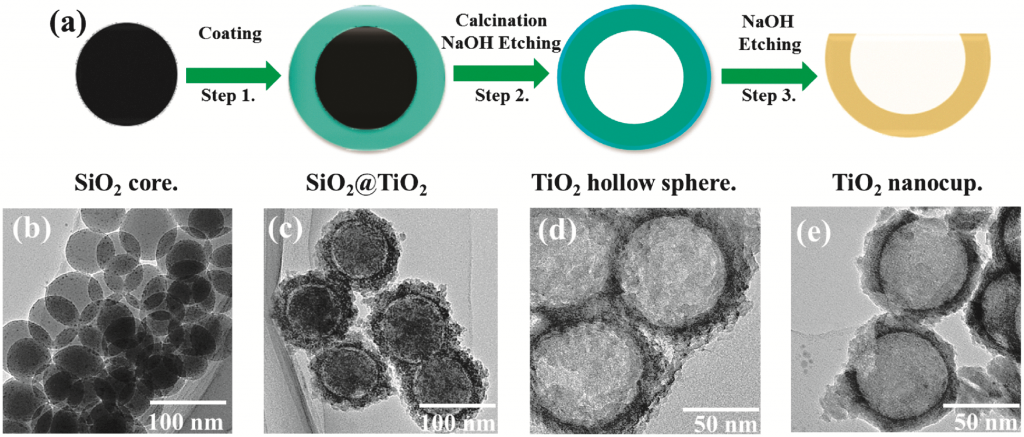
Scientists from the University of Zaragoza in Spain have developed a simple and efficient method of synthesising N-heterocyclic carbene (NHC) gold and silver complexes with the use of an extremely weak base1.
Gold-NHC complexes are commercially important precursors of active, luminescent species that catalyse many useful reactions, such as cycloisomerisation, rearrangement of allylic acetates, C-H activation, carbene transfer, polymerisation, among others. In addition, they have potentially significant applications in the synthesis of new pharmaceuticals and natural products.
Conventional methods of gold-NHC synthesis– the generation of free NHC and the Ag-carbene transfer route– present several logistic and economic limitations, such as the need for an inert atmosphere and the use of additives. These methods are not always efficient, and typically require complicated working conditions in order to produce even moderate yields.
M. Concepción Gimeno and her team’s novel and elegant one-pot synthetic route involves isolating imidazolium salts using [AuCl(tht)] (tht = tetrahydrothiophene) in the presence of a mild base, such as K2CO3, to produce gold-NHC complexes with very high yields (91-94%) over relatively short reaction times (1.5 hours).

Similarly, Gimeno et al. found that, using the same mild base protocol, silver-NHC complexes could also be efficiently synthesised using AgNO3, with vast potential significance in transmetalation.

In both routes, the reactions occur under ambient conditions, eliminating the need to work in an argon atmosphere, and using readily-available technical grade solvents.
Interestingly, a mere few days later, Gimeno et al.‘s groundbreaking work was followed closely and independently by a related Communication from Steven Nolan’s group at the University of St Andrews. In addition to testing a similar methodology, Nolan’s team compared small- and larger-scale reactions, and characterised compounds by 1H and 13C{1H} NMR spectroscopies, as well as by elemental analysis2.
To find out more about these fascinating breakthroughs in organometallics, read these HOT ChemComm articles now for free!
1. Simple and efficient synthesis of [MCI(NHC)] (M = Au, Ag) complexes
Renso Visbal, Antonio Laguna and M. Concepción Gimeno
Chem. Commun., 2013, 49
DOI: 10.1039/C3CC42919A, Communication
2. Straightforward synthesis of [Au(NHC)X] (NHC = N-heterocyclic carbene, X = Cl, Br, I) complexes
Alba Collado, Adrián Gómez-Suárez, Anthony R. Martin, Alexandra M. Z. Slawin and Steven P. Nolan
Chem. Commun., 2013, 49
DOI: 10.1039/C3CC43076F, Communication