Creating chiral layers on a surface is attracting increased attention because of possible application in optical resolution and heterogeneous catalysis. Chiral layers can be achieved by the self assembly of enantiopure molecules on a surface.
Alternatively, achiral molecules can be forced to form chiral surface assemblies by using a chiral building block or by adding a small amount of a chiral auxillary– the “Sergeant-and-Soldiers” effect first described by Mark Green (NYU-Poly) and co-workers in 1989.
In this HOT ChemComm article, Chem Soc Rev Associate Editor David Amabilino from ICMAB-CSIC, Barcelona, ChemComm Associate Editor Steven De Feyter from KU Leuven, and their co-workers have taken this principle a stage further and questioned if the intrinsic chirality of a building block (the “Sergeant”) can be overruled by using a chiral solvent (the “Corporal”).
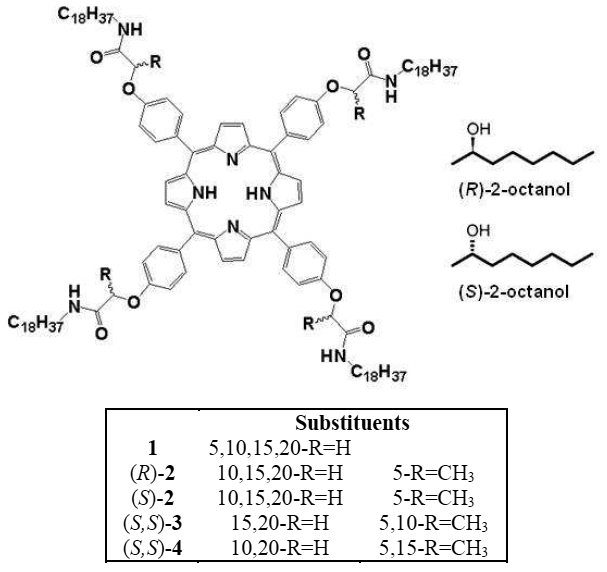
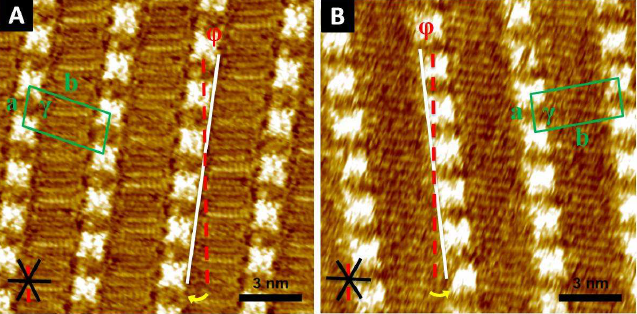
They found that achiral porphyrin 1 could be forced to form chiral monolayers using (S)-and (R)-2-octanol as a solvent. More impressively, they also found that the chirality of the assembly of chiral porphyrins (S)-2 and (R)-2 could be directed using these solvents. The combination of (R)-2 and (S)-2-octanol gave an enantiopure surface assembly, whereas using (R)-2-octanol resulted in a mixture of 2 different domains of opposite chirality. Molecular dynamics simulations indicated that this could be due to hydrogen bonding between the solvent molecules and the amide groups of the porphyrins. If more than one chiral centre was present (3 and 4), the chirality of the molecule was able to dominate the solvent effect.
This is a fascinating report of how a simple, weak interaction with solvent can overcome the inherent chirality of a stereogenic centre. This work could lead to the preparation of bistable systems in which the chirality could be switched with a simple change of solvent.
Download this HOT ChemComm article today!
‘Sergeants-and-Corporals’ principle in chiral induction at an interface
Iris Destoop, Hong Xu, Cristina Oliveras-González, Elke Ghijsens, David B. Amabilino and Steven De Feyter
Chem. Commun., 2013, Advance Article
DOI: 10.1039/C3CC42584C
Cally Haynes is a guest web-writer for ChemComm. She is currently a post doctoral researcher at the University of Southampton, and her research interests include the supramolecular chemistry of anions. When not in the laboratory, she likes travelling and watching football.










