A new family of cobalt-gadolinium cage compounds are highly efficient for low temperature cooling, say European scientists.
Liquid helium is currently used to achieve very low temperatures in a large amount of technology, such as super-conducting magnets that are needed for magnetic resonance imaging scanners. But the world supply of helium is falling, making it more expensive. An alternative method for low temperature cooling is to use demagnetisation of magnetic materials.
Now Richard Winpenny at the University of Manchester and colleagues have synthesised a new family of cobalt-gadolinium cage compounds, creating heterometallic molecular squares that show potential for the creation of magnetic coolants. Winpenny explains that magnetic coolants work because demagnetisation increases the entropy of the material, and this increase in entropy comes from taking heat out of the surroundings.
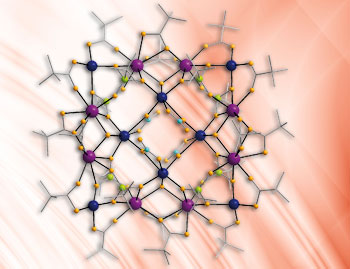
- Cobalt-gadolinium molecular squares act as super coolants
Juergen Schnack at the University of Bielefeld, Germany, an expert in the area of magnetic molecules, comments: ‘most interesting for me is the ability to synthesise such structures in such a great variety as demonstrated by the different grids of this work. This justifies our hopes that compounds with desired properties, for instance a large magnetocaloric effect – where compounds show a large change in temperature with a change in the magnetic field – are achievable.’
The highly anisotropic cobalt(II) ions in these compounds would be expected to have an adverse effect on the magnetocaloric effect, according to the scientists, but anti-ferromagnetic exchange between the octahedral cobalt ions appears to cancel out their contribution.
‘We have a fairly precise understanding of what is needed for a good magnetic coolant,’ says Winpenny. ‘What is surprising is that cobalt(II) complexes shouldn’t meet those requirements, and yet the complexes we’ve studied look interesting. Therefore I think we are going to find a few more surprises along the way and maybe we will need better theory as we proceed.’
Rachel Cooper
To find out more about this ‘cool’ research, download the Chemical Science Edge article for FREE.
Comments Off on Cobalt-gadolinium cages as magnetic refrigerants