Using detective skills that would make Hercule Poirot proud, researchers in the US have solved a longstanding mystery around the absolute configuration of natural product (+)-frondosin B.1
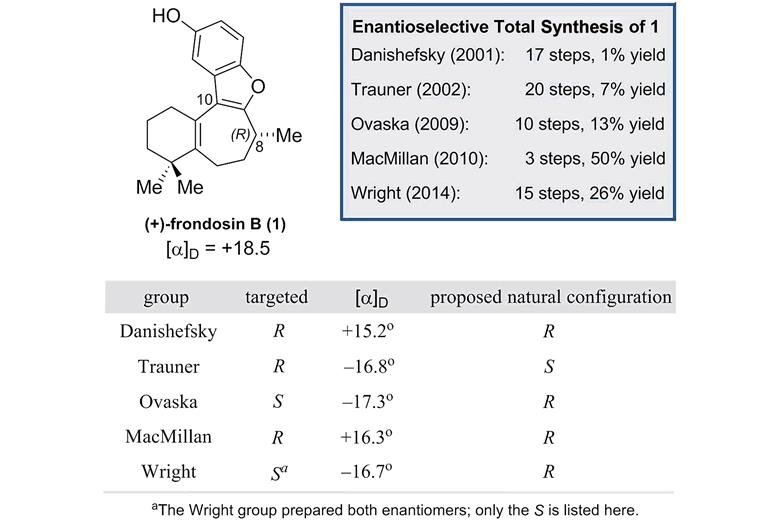
Source: Royal Society of Chemistry Summary of the enantioselective frondosin B syntheses reported to date
(+)-Frondosin B is part of a family of marine sesquiterpenes found in underwater sponges that exhibit anti-inflammatory properties and have potential applications in anticancer and HIV therapy. Starting with Samuel Danishefsky’s route in 2001,2 there have been 5 total syntheses of (+)-frondosin B. However, due to a discrepancy in the optical rotation of the final product during Dirk Trauner’s 2002 synthesis,3 which was observed to have S rather than the expected R configuration, there has been a fierce debate in the synthetic community about the true stereochemistry at C8 in the natural product. After more than decade of attempts by synthetic organic chemists to explain this, particularly focused on different inversion processes, no definitive answer had arisen.
Read the full story by Jason Woolford on Chemistry World.
1 L A Joyce et al, Chem. Sci., 2017, DOI: 10.1039/c7sc04249c (This paper is open access.)
2 M Inoue et al, J. Am. Chem. Soc., 2001, 123, 1878 (DOI: 10.1021/ja0021060)