Scientists in Germany have shed new light on the addition of small molecules to the ubiquitous N-heterocyclic carbenes (NHCs), previously thought to be impossible for NHCs.
Ulrich Siemeling and colleagues have shown that stable NHCs can show strongly enhanced reactivities towards fundamentally important small molecules such as ammonia and carbon monoxide, which is unprecedented for diaminocarbene compounds. The scientists were able to add carbon monoxide to a number of carbene systems, including the simplest stable diaminocarbene, Alder’s C(NiPr2)2 to which they added carbon monoxide. This provided a new entry to the important β-lactam ring systems commonly found in antibiotics.
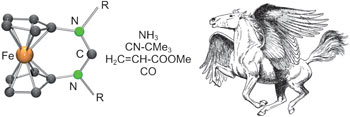
Workhorses taking off: Ferrocene-based N-heterocyclic carbenes undergo reactions with fundamentally important small molecules
This newly discovered reactivity opens the door to an exciting area of synthetically useful carbene chemistry.
Read the Chemical Science Edge article for free online. Have you conquered the impossible? Tell the world by submitting to Chemical Science today.










