UK scientists have developed a methodology that reveals the reactivity and reaction mechanisms of small molecular dipositive ions when they collide with neutral molecules, which could shed light on reactions taking place in interstellar media and in planetary upper atmospheres.
Dipositive ions are highly-energetic species because the two positive charges are located close together in a small molecule. This repulsion makes these species kinetically, as well as thermodynamically, unstable causing them to fragment rapidly forming singly-charged ions. Most small molecules can however hold themselves together for a few seconds to allow it to interact with other atoms and molecules to form monopositive ions.
These metastable ions can often be found in the upper atmospheres of planets. Recent modelling data has suggested that the chemistry of planetary upper atmospheres results from a contribution of these dipositive species (CO22+ on Mars and N22+ on Earth and Saturn’s moon Titan). The data predicts that the N22+ ion could be observed optically. Data collected by the Cassini spacecraft show that the atmosphere of Titan is complex and the chemistry of dipositive ions has been proposed as a mechanism for the formation of hydrocarbons and other large molecules, a reason for which Titan has fascinated chemists and astrobiologists for years.

Titan is one of Saturn's sixty-two moons and has been described as having the richest chemistry in the Solar System
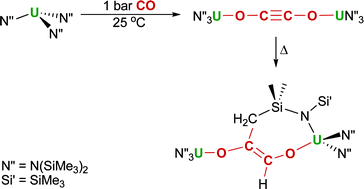
Now, Stephen Price and colleagues at University College London, UK, have developed a position-sensitive coincidence technique (PSCO), which detects pairs of ions following individual dipositive ion collisions with neutral molecules and have applied the technique to show that N22+ reacts with H2 to form NH+ via the initial formation of an [N2H2]2+ collision complex which fragments to NH22+ + N, with the NH22+ then fragmenting to give the observed charged products NH+ + H+.
‘Detecting both the ions from individual reactive events allows for selectively probing the reactions of dications in the presence of other singly-charged ions. We can then determine the identities and velocities of the two product ions from individual dication reactive events,’ says Price. ‘This is a blue-skies area of research, we study these reactions because they are interesting and unusual and we hope will allow us to understand and predict their influence on the chemistry of planetary ionospheres, cometary atmospheres, interstellar space and fusion plasmas, where modelling currently indicates these species play a chemical role,’ he adds.
Stuart Mackenzie, an expert in physical and theoretical chemistry, at Oxford University, UK, comments ‘[This method] provides exquisite detail on a class of chemical reactions which, although exotic on Earth, may play a key role in extraterrestrial environments and the group explore each conceivable mechanism, which might explain the observations and, Sherlock Holmes-style, dismiss them one-by-one as inconsistent with their data until the only one remaining, however improbable, must be the truth.’
The group plan to explore the chemistry of N22+ with other neutral molecules and to study the chemistry of other small dications of relevance to ionospheric and plasma chemistry, such as O22+, CO22+, NO2+ as well as extending the PSCO technique in order to gain more information on the electronic states of the ions involved in the reactions.
Carl Saxton
Read the full story in Price’s Chemical Science Edge article. If you have your own blue-sky research to report, submit to Chemical Science and be seen with the best.
Comments Off on Titan’s ionic atmosphere