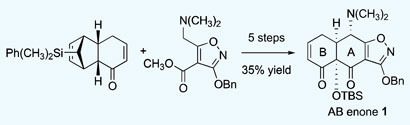
Scientists in the US have developed a sensitive and simple sensor that could be used to detect toxic gases occurring in urban areas.
Gases such as chlorine, sulfur dioxide and ammonia are toxic and are often emitted by industrial processes and agriculture. The gas molecule structures are small and simple, which makes them difficult to differentiate using sensors. Methods to detect such pollutants in urban environments thus require expensive equipment that has to be used in a laboratory.
Eric Kool and colleagues at Stanford University designed a sensor based on the structure of DNA, where base pairs were replaced with one of four fluorescing aromatic monomers. The DNA scaffold gave the sensor a stable structure where the monomers were stacked over each other. Using four sensing molecules in the structures produced a pattern of fluorescence outputs that could be used to differentiate between a mixture of toxic gases.

A combination of three structures could detect and differentiate between eight toxic gases
Read the full Chemistry World news story here
Link to journal Article
DNA polyfluorophores as highly diverse chemosensors of toxic gases
Chi-Kin Koo, Florent Samain, Nan Dai and Eric T. Kool
Chem. Sci., 2011, DOI: 10.1039/c1sc00301a