Marco Di Antonio recently published his first independent research article with ChemComm. We wanted to celebrate this exciting milestone by finding out more about Marco and his research. Check out his #ChemComm1st article: A short peptide that preferentially binds c-MYC G-quadruplex DNA
What are the main areas of research in your lab and what motivated you to take this direction?
My group is interested in developing chemical and biological tools to underpin key chemical and structural changes that DNA undergoes during ageing and diseases development. I have always been fascinated by the relevance of DNA in biology, and to apply fundamental chemistry knowledge to unravel the mysteries behind DNA biology. Indeed, I have been working within this research framework pretty much during my entire career. What attracted me the most to this research topic is the idea that human genomic DNA, which is around 2 meters long, is compacted in a volume of few m2 in a cell. The 3-dimensional architecture that DNA adopts when compacting within a cell nucleus, as well as the chemical modifications that it undergoes to achieve compaction, are key to biological processes such as cell-differentiation, ageing and cancer development. Therefore, we are very interested in understanding what is the role of chemistry and chemical modifications in DNA compaction.
Although we have distinct research projects currently ongoing in my group, they are all aimed at developing chemical biology tools to unravel the fundamental mechanisms that regulate DNA structural dynamics in the context of ageing and diseases, such as cancer. We are particularly interested in non-helical structures that DNA can adopt, and we combine chemistry and biology to investigate how the formation of such non-canonical DNA structures affect human biology.
Can you set this article in a wider context?
It has been almost 70 years since the DNA double-helical structure was described for the first time. Since then, several other DNA structures have been reported. Amongst those, G-quadruplexes have emerged as a stable alternative to the double-helix due to their thermodynamic and kinetic stability. Increasing evidence supports G-quadruplex formation in the context of living cells; therefore, developing chemical tools to target these structures against the canonical DNA double helix is essential.
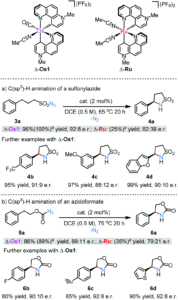
Several molecules that target selectively G-quadruplexes already exists, but there is a chemical need to develop new probes that can target one individual G-quadruplex over the ~700,000 that can form in the human genome. This will allow us to investigate the biology regulated by the targeted G-quadruplex structure and disentangle it from the other G-quadruplexes present in the genome. In this manuscript, we describe a short-peptide that displays preferential selectivity for the G-quadruplex structure present in the promoter region of the oncogene MYC and negligible biding towards other G-quadruplex structures. This has a double impact in the context of G-quadruplex biology: i) it provides a starting point to the design novel peptide-based probes to target specifically other G-quadruplexes besides MYC ii) it will allow biological investigation of the role(s) played by the stabilisation of MYC G-quadruplex, which is relevant in the context of cancer treatment.
What do you hope your lab can achieve in the coming year?
Publishing our first research paper has already been an incredible achievement, considering this has happened a bit more than just a year after starting my group and in the middle of a pandemic! For this, I am particularly thankful to Andrew Jamieson and his PhD student Danielle Morgan who have collaborated with us on this project and have been extremely supportive. For the coming year, it would be great to close a couple of projects that we have currently ongoing but it is a bit too early to predict this! My group started only with a PhD student (Denise Liano) and a PDRA (Aisling Minard), to whom I am very grateful for their relentless work, and we have already come a long way so keeping this trajectory for the next year would be great. We will be expanding in October with two new PhD students joining the team, so I really look forward the vibrant scientific environment that we are establishing within the group, which is helping my creativity significantly!
Describe your journey to becoming independent researcher.
The journey to become an independent academic is not an easy one, I would lie if I said the opposite. But this does not mean that is impossible, and I would encourage anyone reading this to try without even thinking about giving up a single time, if they really want to become independent researchers.
Personally, I have studied for my MSci in Chemistry in Pavia University (2007) and continued for a PhD in Padua University (2011). During my PhD, I almost exclusively worked in a synthetic chemistry laboratory, where I developed some novel G-quadruplex binding small-molecules. After getting my PhD, I was lucky enough to get a postdoctoral offer from Cambridge University to work in the group of Prof. Sir. Shankar Balasubramanian. I have been working in Shankar’s group as a Research Associate for 4 years and then as a Senior Research Associate for 3 more years. My time in Cambridge has been scientifically transformative, I have been moving from synthetic chemistry to biochemistry, cell-biology and genomics. The amount of new skills developed and the extremely intellectually challenging environment that characterises Shankar’s group have been key to develop independent thinking and to start my academic career. It has allowed me to develop a comprehensive view of nucleic acids chemistry and biology that now is at the foundation of my research group.
In December 2017, BBSRC awarded me a David Phillips Fellowship which I have used to start my group at Imperial College Chemistry. Although my research is still very much focused on the chemical biology of nucleic acids, I felt that moving to Imperial has been key to establish my research group in a new environment that is helping me to flourish as an independent scientist.
During my last 3 years of postdoc I started to apply for independent positions, and I am not afraid to share that I failed most of those applications both for fellowships and lectureships. So, my two key pieces of advice to anyone who wants to become independent researcher are: i) give yourself plenty time to make the transition, it will take at least 1 year from applying for a fellowship to get it, even if you get the first one you apply for! So, don’t wait until the end of your contract before giving it a shot; ii) Expect to fail! This is totally normal, and you shouldn’t take it personally, but rather learn from mistakes and improve your applications!
What is the best piece of advice you have ever been given?
The best piece of advice I have been given is from my former post-doc supervisor Prof Sir Shankar Balasubramanian, who always told me: “less is more”. It sounds like a very simple sentence, but as scientists we always tend to overcomplicate things and add extra experiments or extra information in our papers. Being able to disentangle key experiments from non-essential ones, as well as writing up a research paper with the least possible amount of words and jargon, is an essential skill that every scientist needs to keep working on. This is by far the best piece of advice I ever received, and I apply it every time that I design experiments with my group members, or when I write a paper!
Why did you choose to publish in ChemComm?
This is partially connected to my answer for the question above. I love publishing scientific research in the form of a communication, as it forces you to distil out essential and important information from what can be described in more details in supplementary information. Beside the format, ChemComm allows me to quickly and effectively disseminate important proof-of-concept experiments that can be transformative for the chemical community. For us, the findings of a short peptide that shows potential for selective recognition of an individual G-quadruplex were novel and essential to be disseminated quickly. Therefore, I had no hesitation to select ChemComm as a platform to present our first paper. Furthermore, I published with Chem Comm during my post-doc and I was impressed by the quick turnaround of the editors and the smooth submission portal, so it was a very easy decision for us!

Marco obtained his MSci degree in Organic Chemistry from University of Pavia in 2007 and moved to Padua University for his Ph.D in Molecular Sciences under the supervision of Prof. Manlio Palumbo and Prof. Mauro Freccero. Marco obtained his PhD in 2011 and moved to the UK to join Cambridge University. At Cambridge, he worked in the group of Prof. Sir. Shankar Balasubramanian, where he started a scientific transition from synthetic organic chemistry to molecular and cell biology. This scientific approach across boundaries is embedded in his research group that works at the interface between chemistry and biology. In December 2017 Marco was awarded a BBSRC David Phillips Fellowship, which enabled him to move to Imperial College Chemistry to start his research group.
Comments Off on ChemComm Milestones – Marco Di Antonio