Chirality is the concept of non-superimposable mirror images. A simple example of chiral objects are our hands, and in chemistry, molecules are chiral if they have a stereogenic (or asymmetric) centre. Like our hands when it comes to writing or dextrous tasks, chiral compounds are ubiquitous in chemistry, where often only one enantiomer (a chiral isomer) is selective for binding or reactivity. Chiral transition-metal complexes are also desirable for asymmetric catalysis, where the majority of systems have chiral ligands to impart chirality in the complex. The alternative ‘chiral-at-metal’ approach imparts chirality at the metal centre by the selective arrangement of asymmetric, achiral ligands, allowing the metal centre to act as both the stereogenic centre and the reactive centre for catalysis.
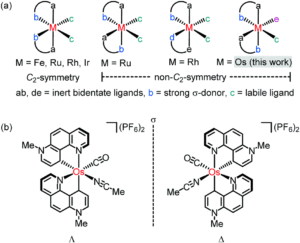
Figure 1: General structure for chiral-at-metal complexes using chelating, achiral ligands (above), and the specific structures of the enantiomers for the new osmium system Os1 (below)
Researchers in Germany have adopted this chiral-at-metal approach for asymmetric catalysis, previously reporting Ir(III), Rh(III), Ru(II) and Fe(II) systems, and have now translated this to the first example of a chiral-at-osmium complex (Figure 1). A new octahedral osmium(II) complex was synthesised (Os1), with two bidentate phenanthrolinium ligands (plus carbonyl and bound acetonitrile ligands) that are coordinated in a non-C2-symmetric fashion to create the stereogenic osmium metal centre. Complex Os1 was initially synthesised as a racemic mixture (rac-Os1), in which the researchers were able to resolve to the individual enantiomers using a chiral auxiliary ligand. This method involved replacing the labile acetonitrile ligand with a chiral auxiliary ligand, (S)-2, to form a mixture of diastereomers Δ-(S)-Os2 and Λ-(S)-Os2, which were separable by solubility. Δ-(S)-Os2 precipitated out of the reaction solution, and was isolated and purified by filtration and washing in a >99:1 diastereomeric ratio (d.r.), and the Λ-(S)-Os2 that remained in solution was purified by column chromatography in a >99:1 d.r. The separated diastereomers were then treated with acid in an acetonitrile solution to replace the chiral auxiliary ligand back to the bound acetonitrile, to give the separated enantiomers Δ-Os1 and Λ-Os1, with >99:1 enantiomeric ratios (Scheme 1).
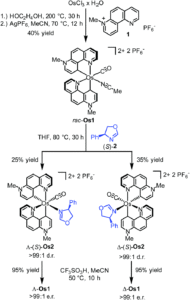
Scheme 1: Synthesis of Os1, starting with formation of the racemic mixture, followed by enantiomeric resolution using a chiral auxiliary ligand (S)-2
The researchers then tested the catalytic activity of the separated enantiomers of Os1 with respect to intramolecular C(sp3)–H aminations that proceed via transition metal nitrenoid intermediates. Δ-Os1 showed catalytic activity for the amination of various nitrene precursor reagents, such as the conversion of sulfonylazides (3) to cyclic sulfonylamides (4) and azidoformates (5) to 2-oxazolidinones (6), tolerating various substrate functional groups (a-c) (Scheme 2). Significantly, the chiral osmium catalyst gave high catalytic yields and enantiomeric purities, particularly in comparison to the previously reported ruthenium analogue (see Scheme 2 for comparative ratios).
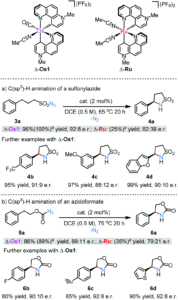
Scheme 2: Catalytic C(sp3)-H aminations using Δ-Os1, with comparisons in activity and enantiomeric purity to the previous ruthenium analogue
Overall, this new chiral-at-osmium complex has shown superior catalytic activity with greater enantiomeric selectivity for intramolecular C(sp3)–H aminations, additionally providing the first examples of catalytic enantioselective ring-closing C-H amination of 2-oxazolidinones. This catalytic activity can be attributed to the labile acetonitrile ligand of Os1, which also proved beneficial for allowing enantiomeric resolution of the initial racemic mixture by substitution with a chiral auxiliary ligand.
To find out more, please read:
Asymmetric Catalysis with Chiral-at-Osmium Complex
Guanghui Wang, Zijun Zhou, Xiang Shen, Sergei Ivlev and Eric Meggers*
Chem. Commun., 2020, 56, 7714-7717
Dr. Samantha Apps is a Postdoctoral Research Associate in the Lu Lab at the University of Minnesota, USA, and obtained her PhD in 2019 from Imperial College London, UK. She has spent the last few years, both in her PhD and postdoc, researching synthetic nitrogen fixation and transition metal complexes that can activate and functionalise dinitrogen. Outside of the lab, you’ll likely find her baking at home, where her years of synthetic lab training has sparked a passion in kitchen chemistry too.











