Researchers at the University of Texas have developed an inventive method to deliver molecules into the cell’s nucleus. Advances in gene therapy and the development of drugs that target DNA, the transcription machinery and other regulatory systems all rely on effective transport of molecules into the nucleus. Furthermore, achieving selective delivery of drugs reduces toxicity to non-target organs while maintaining the therapeutic effect.
Towards this aim, the authors delivered liposomes coated with clusters of gold nanoparticles into the cytoplasm. Laser irradiation of the cells heats the nanoparticles to high temperatures resulting in vapourisation of the water-based cytosol, and the transient formation of nanobubbles. The effect of this is an increase in the porosity of the nuclear envelope, enabling the transfer of various macromolecules from the cytoplasm into the nucleus. The authors describe this technique as ‘nanomechanical transduction’ because it is hypothesised that the mechanical effects brought on by the rapid growth and collapse (20 – 50 ns lifetimes) of the bubbles is responsible for the observed increase in porosity.
Local heating of gold nanoparticles and the subsequent formation of nanobubbles occurs due to ‘plasmon resonance’, whereby an electromagnetic field interacts with gold on the surface of the liposome and drives free-electron oscillation in resonance with the incident laser.
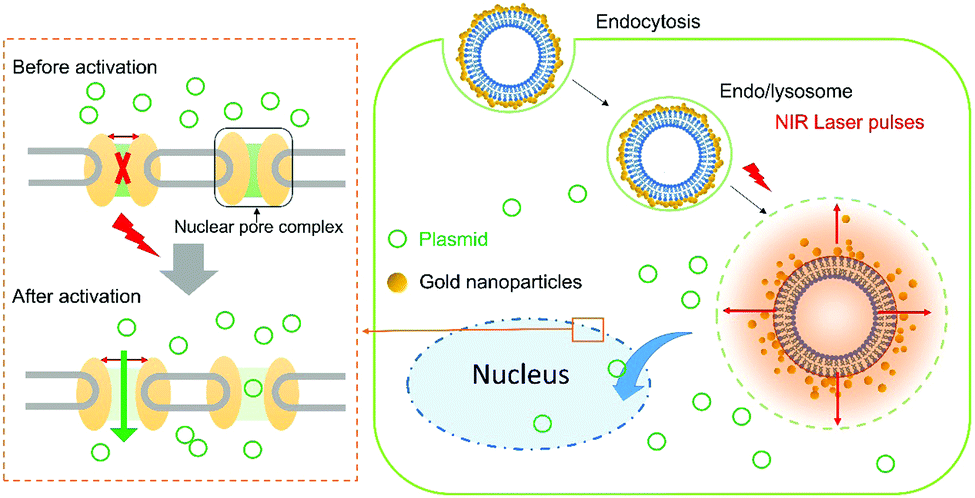
A diagram showing nanomechanical transduction. A gold-coated liposome enters the cell and, upon activation by a laser pulse, creates nanobubbles and mechanical disruption within the cell, resulting in increased permeability of the nuclear membrane.
As a proof-of-concept the authors investigated whether nanomechanical transduction can improve the nuclear localisation of two different types of macromolecule: a dextran polymer labelled with a fluorescent dye, and a plasmid encoding the green fluorescent protein. In the first experiment, cells containing the dextran polymer were incubated with plasmonic liposomes and subjected to a near-infrared laser pulse. Up to 70% fluorescence intensity was observed in the nucleus compared to the cytoplasm, far exceeding the result from control experiments using electroporation to increase cell membrane permeability. In a similar way, nanomechanical transduction resulted in a 2.7 fold increase in the expression of the green-fluorescent protein compared to using electroporation, demonstrating efficient delivery of the plasmid into the nucleus.
The authors entitle their paper ‘rock the nucleus’ and, unintentional reference or not, I think a Casbah (one meaning is the central keep, or citadel, of a walled city) is a rather fitting analogy for the nucleus: the command post of the cell, and safeguard of genetic information. The authors of this work offer a sophisticated yet general method for molecules to breach the walls.
To find out more please read:
Xiuying Li, Peiyuan Kang, Zhuo Chen, Sneha Lal, Li Zhang, Jeremiah J. Gassensmith and Zhenpeng Qin.
Chem. Commun., 2008, Advance Article
DOI: 10.1039/c7cc09613e
Zoë Hearne is a PhD candidate in chemistry at McGill University in Montréal, Canada, under the supervision of Professor Chao-Jun Li. She hails from Canberra, Australia, where she completed her undergraduate degree. Her current research focuses on transition metal catalysis to effect novel transformations, and out of the lab she is an enthusiastic chemistry tutor and science communicator.












 ____________________________________________________
____________________________________________________