A rotaxane is a mechanically-interlocked molecule that consists of one or more rings trapped on a linear unit, the thread, by two bulky constituents, the stoppers. Remarkably, the ring components are not covalently attached to the dumbell component, rather a mechanical bond is present that intrinsically links the components of the molecule and prevents their dissociation without the cleavage of one, or more, covalent bonds. The synthesis of these interlocked molecules is of much interest to chemists today as a means of not only synthetically mimicking molecular geometries found in nature, but also, and perhaps more interestingly, as a means of exploiting the emergent properties imparted as a result of the mechanical bond for function as catalysts, motors and sensors, to name but a few examples.
The synthesis of rotaxanes is analogous to Goldilock’s quest to find the perfect bowl of porridge or the bed that is just right – finding the correctly-sized macrocycle to thread a rotaxane dumbbell is also a game of too big, too small or just right. Historically speaking, many of the rotaxanes reported thus far achieve “just right” by providing a steric bulk to the dethreading of the two components by simply increasing the size of the rotaxane stoppers. This is a valid approach, however it would also be advantageous in terms of synthetic ease and the possibility of introducing diversity to the rotaxane library if we could move away from big macrocycles and the necessary bulky stoppers, to smaller stoppers that allow for much smaller macrocycles.
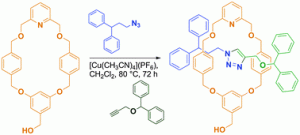
But – how small is too small? Sometimes a macrocycle is just not big enough. Steve Moratti and James Crowley of the University of Otago, and coincidentally where i began my foray into chemistry, have set out to study just this – what is the smallest macrocycle that can be incorporated into a [2]rotaxane synthesized using the highly efficient Cu(I)-catalyzed Huigsen 1,3-dipolar cycloaddition active metal template approach developed by the Leigh group( JACS, DOI:10.1021/JA056903F).
To date, the smallest macrocycle that has been utilized in the synthesis of such rotaxanes is a 26-membered ring that generates [2]rotaxanes in high yields (DOI; 10.002/ANIE.201100415). Moratti and Crowley took this exploration a step further and studied the possibility of rotaxanation using even smaller 22- and 24-membered rings. One of the biggest advantages of moving towards smaller rotaxanes is the greater ability with which they can be functionalized over their larger analogues. Smaller macrocycles and less chemically-complicated stopper groups can be functionalized much more readily, as demonstrated in this work by the incorporation of a free hydroxymethyl group into the macrocycle and the use of unfunctionalized phenyl groups in the stopper components.
This study determined that the limit for rotaxanation was the larger of the two rings, with a [2]rotaxane forming in 70% yield – read the article in full for free* to find out more!
CuAAC “click” active-template synthesis of functionalised [2]rotaxanes using small exo-substituted macrocycles: how small is too small?
Asif Noor, Warrick K. C. Lo, Stephen C. Moratti and James D. Crowley
DOI: 10.1039/C4CC03077J
You may also like to have a look at this Feature Article by Edward Neal and Stephen Goldup* from Queen Mary University, which reviews some of the less discussed consequences of mechanical bonding for the chemical behaviour of rotaxanes, and their application in synthesis
*Access is free through a registered RSC account – click here to register
 About the web writer
About the web writer
Anthea Blackburn is a guest web writer for Chemical Science. Anthea is a graduate student hailing from New Zealand, studying at Northwestern University in the US under the tutelage of Prof. Fraser Stoddart (a Scot), where she is exploiting supramolecular chemistry to develop multidimensional systems and study the emergent properties that arise in these superstructures. When time and money allow, she is ambitiously attempting to visit all 50 US states before graduation.
 Rachel completed her PhD at the University of Melbourne and then worked as a postdoctoral fellow and group leader at Berlin’s Hahn-Meitner Institute and the Max-Planck Institute of Colloids and Interfaces in Germany. She returned to Australia in 2003 to take up an Australian Research Council Fellowship. Since 2008, Rachel has held a joint appointment between the University of Melbourne and CSIRO as an Associate Professor and Reader in the School of Chemistry and as a CEO Science Leader in the division of Materials Science and Engineering. She currently leads an Advanced Porous Materials research group which consists of postdoctoral fellows and PhD students at both the Univeristy of Melbourne and CSIRO.
Rachel completed her PhD at the University of Melbourne and then worked as a postdoctoral fellow and group leader at Berlin’s Hahn-Meitner Institute and the Max-Planck Institute of Colloids and Interfaces in Germany. She returned to Australia in 2003 to take up an Australian Research Council Fellowship. Since 2008, Rachel has held a joint appointment between the University of Melbourne and CSIRO as an Associate Professor and Reader in the School of Chemistry and as a CEO Science Leader in the division of Materials Science and Engineering. She currently leads an Advanced Porous Materials research group which consists of postdoctoral fellows and PhD students at both the Univeristy of Melbourne and CSIRO.