This ChemComm Feature Article by Chuo Chen’s group, based at the Southwestern Medical Center, University of Texas focuses on biosynthesis and total synthesis of cyclic pyrrole-imidazole dimers. Pyrrole-imidazole alkaloids are secondary metabolites which are found exclusively in marine sponges. They have very unique structures and attractive biological properties. Part of what makes these molecules so interesting is the fact that they contain many functional groups and are highly populated with nitrogen atoms. Pyrrole-imidazole alkaloids often have polycyclic skeletons which make them ideal platforms to work on in the development of new synthetic ideas and methodologies.
 Many pyrrole-imidazole alkaloids have been tested and determined to have promising biological properties such as anticancer, antimicrobial, antiviral or immunosuppressive activities. Although this is the case, much work still needs to be carried out to determine the full biological profile of pyrrole-imidazole alkaloids.
Many pyrrole-imidazole alkaloids have been tested and determined to have promising biological properties such as anticancer, antimicrobial, antiviral or immunosuppressive activities. Although this is the case, much work still needs to be carried out to determine the full biological profile of pyrrole-imidazole alkaloids.
Another aspect of pyrrole-imidazole alkaloids which still contains unknowns is the biosynthetic pathway; a range of biosynthetic pathways have been suggested but the complete route has not yet been fully determined. It is agreed that the main stages of the biosynthesis are catalysed by cyclases and oxidases but the exact enzymes have not been identified. A number of interesting hypotheses are highlighted and discussed in this review including work from Faulkner and Clardy who isolated the first dimeric pyrrole-imidazole alkaloid, sceptrin, in 1981.
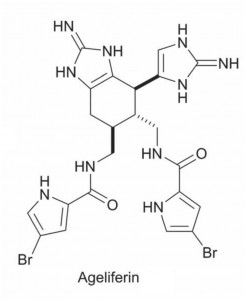
As well as summarising different biosynthetic routes to these intriguing compounds the authors also discuss synthetic strategies. Numerous groups have successfully synthesised different pyrrole-imidazole dimers and highlights of this section include Baran’s work synthesising a number of different dimers and Chen’s own work which involves developing a biomimetic approach for the synthesis of ageliferins. Chen’s synthesis contains an oxidative radical cyclisation as the key step to give the ageliferin core skeleton. The group have successfully synthesised a range of ageliferins using this adaptable approach.
To download the full article for free* click the link below:
Dimeric pyrrole-imidazole alkaloids: synthetic approaches and biosynthetic hypotheses
Xiao Wang, Zhiqiang Ma, Xiaolei Wang, Saptarshi De, Yuyong Ma and Chuo Chen
DOI: 10.1039/c4cc02290d
*Access is free until the 12.07.14 through a registered RSC account – click here to register










