Optical antennas made of gold nanoparticles can enhance the sensitivity of photoluminescence and vibrational spectroscopy, according to research recently published in Chemical Science.
In traditional microscopy and spectroscopy, components such as lenses, mirrors and diffractive elements are used to control and focus the optical radiation. This relies on the wave nature of the radiation and means the smallest volume to which the radiation can be localised, and so the technique’s sensitivity, is limited by diffraction.
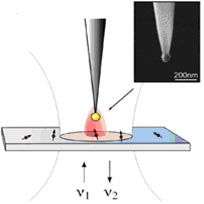
Now Lukas Novotny and colleagues at the University of Rochester, USA, have taken inspiration from radio wave manipulation and designed an optical antenna that can boost the interaction between light and the particle being studied. The fluorescence of a single molecule can be enhanced by more than a factor of 10 using this technique. The optical antenna, which consists of a single colloidal gold nanoparticle on the end of a pointed dielectric fibre, replaces a conventional focusing lens or objective, so the incoming light can be focused to dimensions smaller than the diffraction limit.
As well as improving chemical and biological sensing, the technique holds promise for resolving open questions in surface enhanced Raman scattering and fluorescence, says Novotny.
Read the Edge article to find out more. And to put the focus on your own exciting research, submit today to Chemical Science.