Researchers from the Biophotonics Research Unit of Gloucestershire Hospitals NHS Foundation Trust, in collaboration with scientists from the University of Strathclyde and the Rutherford Appleton Laboratory, have published results of their exploration into surface enhanced spatially offset Raman spectroscopy (SESORS) imaging, a new technique combining surface enhanced Raman scattering (SERS) with spatially offset Raman spectroscopy (SORS).
They achieved, for the first time, imaging of SERS signals recovered from a depth of 20 mm in tissues, opening the way for sampling a number of disease conditions in the same organ at the same time. This could potentially lead to a new methodology for enhanced personalised treatment plans to be developed in realtime.
The Chemical Science Edge article has been highlighted in the following RSC press release:
“UK scientists have explored surface enhanced spatially offset Raman spectroscopy (SESORS) imaging and found that multiplexed surface enhanced Raman scattering (SERS) signals have been recovered non-invasively from a depth of 20 mm in tissues for the first time and reconstructed to produce a false colour image. This approach could be adapted into a clinical setting for disease diagnosis, say the researchers.
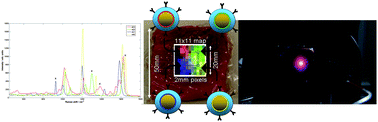
The team injected four unique ‘flavours’ of SERS nanoparticles (NPs) into a 20 x 50 x 50 mm porcine tissue block at the corners of a 10 mm square. A transmission Raman data cube was acquired over an 11 x 11 pixel grid made up of 2 mm steps. The signals were reconstructed using the unique peak intensities of each nanoparticle. A false colour image of the relative signal levels was produced, demonstrating the capability of multiplexed imaging of SERS nanoparticles using deep Raman spectroscopy.
A secondary but no less significant achievement was to demonstrate that Raman signals from SERS nanoparticles can be recovered non-invasively from samples 45–50 mm thick. This is a significant step forward in the ability to detect and identify vibrational fingerprints within tissue, say the team.
The prospects for SESORS as a medical tool are significant, say the researchers. There are numerous applications where this approach could have a major impact on rapid specific diagnosis, patient specific treatment selection and treatment monitoring. However, the greatest hurdle will be introducing nanoparticles into the body without fully understanding their excretion mechanism or long term accumulation sites and whether this is likely to have detrimental effects.”
Reference:
N Stone, M Kerssens, G R Lloyd, K Faulds, D Graham and P Matousek, Chem. Sci., 2011, DOI: 10.1039/c0sc00570c
Comments Off on Surface enhanced spatially offset Raman spectroscopic imaging – the next dimension