A helical framework around an electrophilic centre is an effective control element for the stereoselective addition of nucleophiles, according to European scientists.
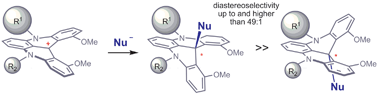
Stereoselective additions of nucleophiles to trivant carbon atoms are common organic reactions. Chemists often rely on stereogenic elements close to the reactive centres to influence the stereochemical outcome. For example, nucleophiles can preferentially add to one of the two diastereotopic faces of a carbenium ion when there is an α-stereocentre.
Now Jérome Lacour, at the University of Geneva, Switzerland, and colleagues have shown that nucleophiles can also distinguish between the diastereotopic faces of chiral cationic helicenes. Using hydride and organolithium reagents, the group achieved diastereomeric ratios higher than 49:1.
More information can be found in Professor Lacour’s Edge article.