Otto Warburg was a rather interesting man; not only was he a Nobel prize winner, he was BFFs with Einstein, served in the cavalry in the war, and also insisted using his own tea bags when he went out for a cuppa. Otto also observed that cancerous tissues consumed rather large amount of glucose compared to non-cancerous tissues and also had high rates of aerobic glycolysis. These observations, now known as the Warburg effect, are now recognised as some of the hallmarks of cancer.
A recent Chemical Science Minireview by Emilia Calvaresi and Paul Hergenrother focuses on the current progress and future directions of exploiting the Warburg effect by targeting it for cancer treatment. One potential strategy is glycoconjugation; simply put, the linking of a drug to a sugar. Unfortunately, however, it is not as simple as dipping a fun-size Mars bar in some cisplatin.
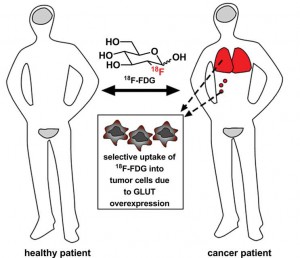
The strategy for glycoconjugation of anticancer drugs was inspired by the use of 18F-FDG, a radiolabeled glucose analogue used to visualise tumours.
Like a rather strange cake recipe book, this review discusses ways to make sugary, anticancer conjugates– it does mention sugar and mustard at one point– but more seriously, it explains the developments in this anticancer approach, the difficulties and the lessons learned, in a clear and comprehensible way.
Since the first report of glycoconjugated anticancer drugs in 1995, this field has rapidly developed to the point that one conjugate (glufosfamide) is already in advanced trials, and Calvaresi and Hergenrother discuss this, as well as other anticancer glycoconjugates that are in development.
Importantly, Calvaresi and Hergenrother recognise that, for these glycoconjugated anticancer compounds to be successful, there are outstanding issues that need addressed, i.e., what is the best way to make the cancer ‘eat up’ these conjugates? Do you offer it the dark chocolate or the milk chocolate? Which position on the sugar should be substituted? Are their more effective sugars? What’s the best way to test the efficacy, i.e., how do we measure how much the cancer has eaten, and if it likes it?
The authors conclude that this field has a great deal of potential but, just like any new confectionery, it needs to be rigorously developed at each stage for optimum customer satisfaction.
Read this HOT Chem Sci Minireview in full!
Glucose conjugation for the specific targeting and treatment of cancer
Emilia C. Calvaresi and Paul J. Hergenrother
Chem. Sci., 2013, Advance Article
DOI: 10.1039/C3SC22205E
Sarah Brown is a guest web-writer for Chemical Science. Sarah hung up her lab coat after finishing her PhD and post-doctorate in nanotechnology for diagnostics and therapeutics, to become an assistant editor at the BMJ Publishing Group. When not trying to explain science through ridiculous analogies, you can often find her crocheting, baking or climbing, but not all at once.
Comments Off on Cancer in a candy shop


-429---NS_tcm18-221958.jpg)
-429---NS_tcm18-221955.jpg)
-429---NS_tcm18-221866.jpg)