Lift your right foot off the floor and make clockwise circles with it. Managing comfortably? Now draw a ‘6’ in the air with your right hand – what’s happened to your foot? It’s all gone a bit wrong, hasn’t it? See, it isn’t that easy to do two different things at once. Another example of this is the dual labelling of proteins; however, Stephen Caddick and colleagues appear to have got on top of this, as reported in their recent paper in Chemical Science.
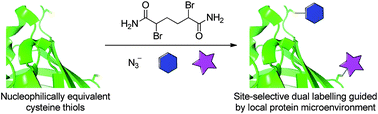
The dual labelling of proteins has the potential to enable studies of protein structures and the construction of theranostics, for example; however, proteins can be complex and modifications are often restricted to the N- and C-termini, limiting their usefulness. Modification of non-terminal positions is tricky and can be slow, expensive and unrewarding. Caddick and colleagues demonstrate a novel approach to site-selective labelling of proteins, which yields a dual-labelled product by the introduction of two cysteine mutants into the sequence, which are converted by a single chemical reagent into two distinct products for modification. One residue, with an accessible alpha-proton, readily forms dehydroalanine, and the other residue persists, by shielding, as a sulfonium that undergoes chemoselective ring opening by reaction with an azide group. Both groups can then be further labelled orthogonally by the desired molecules.
The group demonstrated their technique by modifying GFP (green fluorescent protein). After incorporation of the cysteine mutants and treatment with a chemical reagent (2, 5-dibromohexanediamide), further treatment with sodium azide generated dual modified GFP. Further reaction with an alkyne modified dye and mercaptoethanol yielded a rhodamine dye and thiol-labelled protein.
The researchers have demonstrated a site- and chemoselective method, which they say offers a facile and generally accessible technique for dual labelling. And now they’ve got to grips with that, I pose the ultimate test: to come up with a facile method for standing on one leg and drawing a ‘6’ – trying to do that and type this blog has also been pretty challenging.
Once you’ve tried out some one-legged multi-tasking, sit back down and read this Open Access Edge Article to see what Sarah’s talking about:
A novel approach to the site-selective dual labelling of a protein via chemoselective cysteine modification
Ramiz I. Nathani, Paul Moody, Vijay Chudasama, Mark E. B. Smith, Richard J. Fitzmaurice and Stephen Caddick
Chem. Sci., 2013, 4, 3455-3458
DOI: 10.1039/C3SC51333E
Sarah Brown is a guest web-writer for Chemical Science. Sarah hung up her lab coat after finishing her PhD and post-doctorate in nanotechnology for diagnostics and therapeutics and now works in scientific publishing. When not trying to explain science through ridiculous analogies, you can often find her crocheting, baking or climbing, but not all at once. All views are her own.