The movement of molecules in and out of cells is fundamental to the chemistry of life. The process is facilitated by other molecules, specifically designed (or perhaps evolved!) to assist in transportation across the cell wall. It is a complicated process and remains poorly understood yet it is known that disruption of the process is linked to many debilitating diseases, such as cystic fibrosis. As a result, there is significant interest in increasing our understanding of the chemistry involved. Only by doing this will it be possible to create treatments which could, for example, use synthetic carriers to improve transportation in cases where it is restricted.
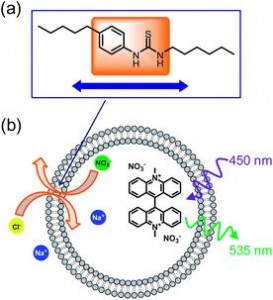
(a) Varying the position of the anion-binding group (b) Transport activities in synthetic vesicles are measured via the quenching of lucigenin fluorescence by incoming chloride.
Insight into transport of anions across cell membranes is reported in back-to-back Edge Articles in Chemical Science by Philip Gale and his network of collaborators at the University of Southampton, the University of Sydney, the University of Ljubljana and the NMR centre at the National Institute of Chemistry in Slovenia, and the University of Bristol. In the first paper, Gale’s group and the team of Anthony Davis at Bristol address a new principle, which they refer to as ‘lipophilic balance’. Anion carriers must have an affinity for the ions they need to transport but, as they discover, the location of that anion receptor within the molecule is also critical. A central or balanced location with the transport carrier results in a much faster transportation of the anion.
In the second paper, Gale and co-workers team up with Katrina Jolliffe (Sydney), Janez Plavec (Slovenia), and their respective groups to examine transport of a poorly studied anion – sulphate. To do this, they explain how they developed a new method based upon sulphur NMR spectroscopy. This has allowed them to monitor sulphate transportation through differences they observe, both inside and outside of the vesicle. Furthermore, they establish that the movement is assisted by small cyclic molecules that are able to wrap around the sulphate.
The work is also reported through an exciting new website called Kudos – a prototype site which works with selected authors to test the effect on download and social media activity of collating and creating multimedia and additional metadata around articles. The pilot project, supported by the Royal Society of Chemistry and other publishers, is designed to help scientists enhance the impact of their published work, increasing visibility and discoverability. This is achieved by providing a framework to include supplementary information which is often not possible to include in a journal submission, such as a discussion of the work in lay terms, the wider impact of the work and supplementary information such as videos. The greater accessibility of the work therefore allows the researchers to engage a wider audience and maximise their impact.
Access these two Chemical Science Edge Articles on Kudos today – FREE to read!
Lipophilic balance – a new design principle for transmembrane anion carriers
Hennie Valkenier, Cally J. E. Haynes, Julie Herniman, Philip A. Gale and Anthony P. Davis
Chem. Sci., 2014, Advance Article
DOI: 10.1039/C3SC52962B
Synthetic transporters for sulfate: a new method for the direct detection of lipid bilayer sulfate transport
Nathalie Busschaert, Louise E. Karagiannidis, Marco Wenzel, Cally J. E. Haynes, Neil J. Wells, Philip G. Young, Damjan Makuc, Janez Plavec, Katrina A. Jolliffe and Philip A. Gale
Chem. Sci., 2014, Advance Article
DOI: 10.1039/C3SC52006D
Ruaraidh McIntosh is a guest web-writer for Chemical Science. His research interests include supramolecular chemistry and catalysis. When not working as a Research Fellow at Heriot-Watt University, Ruaraidh can usually be found in the kitchen where he has found a secondary application for his redoubtable skills in burning and profanity.