This month sees the following articles in Chemical Science that are in the top ten most accessed:-
Why not take a look at the articles today and blog your thoughts and comments below.
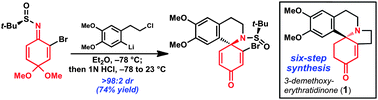
A synthesis of strychnine by a longest linear sequence of six steps
David B. C. Martin and Christopher D. Vanderwal
Chem. Sci., 2011, 2, 649-651, DOI: 10.1039/C1SC00009H, Edge Article
Transition metal-catalyzed cross coupling with N-acyliminium ions derived from quinolines and isoquinolines
Thomas J. A. Graham, Jason D. Shields and Abigail G. Doyle
Chem. Sci., 2011, Advance Article, DOI: 10.1039/C1SC00026H, Edge Article
Dialkylbiaryl phosphines in Pd-catalyzed amination: a user’s guide
David S. Surry and Stephen L. Buchwald
Chem. Sci., 2011, 2, 27-50, DOI: 10.1039/C0SC00331J, Perspective
Enantioselective silver-catalyzed propargylation of imines
Hanna M. Wisniewska and Elizabeth R. Jarvo
Chem. Sci., 2011, 2, 807-810, DOI: 10.1039/C0SC00613K, Edge Article
Chemoselective enrichment for natural products discovery
Antoinette Y. Odendaal, Darci J. Trader and Erin E. Carlson
Chem. Sci., 2011, 2, 760-764, DOI: 10.1039/C0SC00620C, Edge Article
Gold(i)-catalyzed intermolecular (4 + 2) cycloaddition of allenamides and acyclic dienes
Hélio Faustino, Fernando López, Luis Castedo and José L. Mascareñas
Chem. Sci., 2011, 2, 633-637, DOI: 10.1039/C0SC00630K, Edge Article
Palladium-catalyzed coupling of functionalized primary and secondary amines with aryl and heteroaryl halides: two ligands suffice in most cases
Debabrata Maiti, Brett P. Fors, Jaclyn L. Henderson, Yoshinori Nakamura and Stephen L. Buchwald
Chem. Sci., 2011, 2, 57-68, DOI: 10.1039/C0SC00330A, Edge Article
Total synthesis of diazonamide A
Robert R. Knowles, Joseph Carpenter, Simon B. Blakey, Akio Kayano, Ian K. Mangion, Christopher J. Sinz and David W. C. MacMillan
Chem. Sci., 2011, 2, 308-311, DOI: 10.1039/C0SC00577K, Edge Article
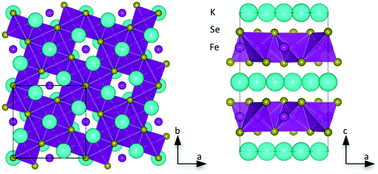
Coordination chemistry of group 13 monohalides
Dragoslav Vidovic and Simon Aldridge
Chem. Sci., 2011, 2, 601-608, DOI: 10.1039/C0SC00508H, Minireview
Stable singlet carbenes as mimics for transition metal centers
David Martin, Michele Soleilhavoup and Guy Bertrand
Chem. Sci., 2011, 2, 389-399, DOI: 10.1039/C0SC00388C, Perspective
Fancy submitting an article to Chemical Science ? Then why not submit to us today or alternatively email us your suggestions.