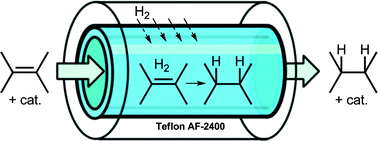
Spanning a vast field of chemistry and biology, it is hard not to notice the flurry of activity and excitement surrounding chemical biology research at the moment. We are seeing chemistry being cleverly applied to biology, in an attempt to understand and answer important biological questions, by directly probing living systems at the chemical level. Areas such as proteomics, glycobiology, combinatorial chemistry, RNA/DNA, microarrays, proteins, peptides (and many, many more!) are becoming prominent topics in the general chemistry literature.
Here at Chemical Science, we have two fantastic Associate Editors, who handle all submissions in the chemical biology and bioorganic fields; Professor Thomas Carell (Ludwig-Maximilians-Universtät München, Germany) and Professor Benjamin F Cravatt (Scripps, USA). We also have the full support from our dynamic team of chemical biology experts on the Chemical Science Advisory Board; Ben Davis, Linda Hsieh-Wilson, Scott Miller, Paul Reider, Oliver Seitz, Weihong Tan and Jason Chin.
| Why not submit your own exciting chemical biology research to Chemical Science today? Our Associate Editors, Professors Thomas Carell and Benjamin Cravatt, handle all submissions within the chemical biology field and eagerly await your next exciting, high impact submission! |
|
We’ve already published some excellent articles in the field of chemical biology and below we’ve given you a little taster. As a reminder, all articles published in Chemical Science are free to access until the end of 2011, so go ahead and enjoy the free content!
| Comparative bioinformatics analysis of the mammalian and bacterial glycomes Alexander Adibekian, Pierre Stallforth, Marie-Lyn Hecht, Daniel B. Werz, Pascal Gagneux and Peter H. Seeberger Chem. Sci., 2011, 2, 337-344 |
| The programming role of trans-acting enoyl reductases during the biosynthesis of highly reduced fungal polyketides Mary N. Heneghan, Ahmed A. Yakasai, Katherine Williams, Khomaizon A. Kadir, Zahida Wasil, Walid Bakeer, Katja M. Fisch, Andrew M. Bailey, Thomas J. Simpson, Russell J. Cox and Colin M. Lazarus Chem. Sci., 2011, 2, 972-979 |
| Development and evaluation of new cyclooctynes for cell surface glycan imaging in cancer cells Henning Stöckmann, André A. Neves, Shaun Stairs, Heather Ireland-Zecchini, Kevin M. Brindle and Finian J. Leeper Chem. Sci., 2011, 2, 932-936 |
| Fragment screening against the thiamine pyrophosphate riboswitch thiM Elena Cressina, Liuhong Chen, Chris Abell, Finian J. Leeper and Alison G. Smith Chem. Sci., 2011, 2, 157-165 |
| DNA-programmed spatial screening of carbohydrate–lectin interactions Christian Scheibe, Alexander Bujotzek, Jens Dernedde, Marcus Weber and Oliver Seitz Chem. Sci., 2011, 2, 770-775 |
| Dissecting tunicamycin biosynthesis by genome mining: cloning and heterologous expression of a minimal gene cluster Filip J. Wyszynski, Andrew R. Hesketh, Mervyn J. Bibb and Benjamin G. Davis Chem. Sci., 2010, 1, 581-589 |
| End-functionalized glycopolymers as mimetics of chondroitin sulfate proteoglycans Song-Gil Lee, Joshua M. Brown, Claude J. Rogers, John B. Matson, Chithra Krishnamurthy, Manish Rawat and Linda C. Hsieh-Wilson Chem. Sci., 2010, 1, 322-325 |
| Discovery of an orexin receptor positive potentiator Jiyong Lee, M. Muralidhar Reddy and Thomas Kodadek Chem. Sci., 2010, 1, 48-54 |
One last thing, you may also be interested to know that the following ISACS5 conference is coming up soon too:-
– Call for posters – deadline 27 May 2011
– Early bird registration – deadline 27 May 2011
– Registration – deadline 24 June 2011














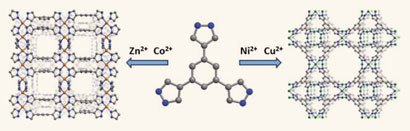


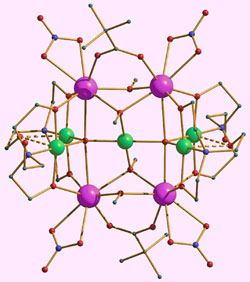 Scientists have laid the foundations for a high-performance ‘molecular fridge’ capable of reaching temperatures within a few thousandths of a degree of absolute zero (0K) with a high degree of efficiency. Such ultracoolers could have applications in areas such as ultra-low temperature physics, where alternative technologies such as those that rely on expensive and rare helium-3 could be unsuitable or too costly.
Scientists have laid the foundations for a high-performance ‘molecular fridge’ capable of reaching temperatures within a few thousandths of a degree of absolute zero (0K) with a high degree of efficiency. Such ultracoolers could have applications in areas such as ultra-low temperature physics, where alternative technologies such as those that rely on expensive and rare helium-3 could be unsuitable or too costly.