Strychnine, best known as a poison but also used medicinally as a stimulant, can now be synthesised in just six steps, say US scientists.
Christopher Vanderwal and his team from the University of California, Irvine created four new carbon-carbon bonds and a carbon-oxygen bond in four steps on the way to making strychnine.
‘Until recently, the fastest synthesis was completed by Viresh Rawal [from The Ohio State University, US] in 14 steps,’ says David MacMillan, an expert in organocatalysis from Princeton University in the US. ‘For a molecule of this complexity, 14 steps is an amazing accomplishment. To be able to complete this in just six steps is simply incredible and something I didn’t necessarily think would ever be possible.’
Defining the shortest route to complex, useful molecules is an important step in uncovering the most efficient way to produce these targets, says Vanderwal. ‘While the overall efficiency of our route isn’t any better than previous routes, the number of chemical operations needed is less than any predecessor,’ he says.
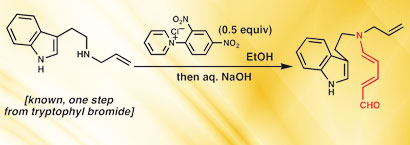
The synthesis began with a pyridinium ring opening reaction to form donor-acceptor dienes known as Zincke aldehydes
The team began with a century-old pyridinium ring opening reaction called the Zincke reaction – named after German chemist Theodor Zincke – in which a pyridine is transformed into a pyridinium salt by reaction with 2,4-dinitrochlorobenzene and a primary amine. This led to the formation of donor-acceptor dienes known as Zincke aldehydes. The next steps involved an intramolecular Diels-Alder reaction, a ruthenium catalysed hydrosilylation and a rearrangement-intramolecular conjugate addition leading to an aldehyde that was then converted to strychnine
‘Strychnine is the oldest and perhaps most famous “celebrity molecule”,’ remarks MacMillan. ‘Total syntheses of this molecule are among the most famous of all completed to date. As such, the field of total synthesis uses strychnine synthesis as a molecular benchmark.’
‘Its first synthesis by Robert Burns Woodward, reported in 1954, stands as an absolute classic,’ adds Vanderwal. ‘Woodward’s pioneering achievement and the numerous syntheses since then have taught us about synthesis strategy, biosynthesis, reaction design, asymmetric catalysis, and more.’
Vanderwal sees the ability to build up complex molecular scaffolds in very few steps using their predictable reactivity pattern as the way forward. ‘It need not be a Zincke aldehyde, and the targets need not be indole alkaloids,’ he says. ‘The onus is on organic chemists to increase our ability to make molecules in the most efficient way possible.’
Elinor Richards
Link to Chemical Science article:-
A synthesis of strychnine by a longest linear sequence of six steps
David B. C. Martin and Christopher D. Vanderwal, Chem. Sci., 2011
DOI: 10.1039/c1sc00009h