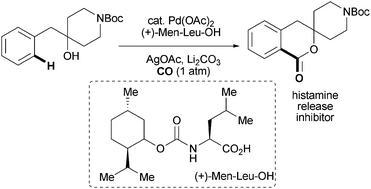
A rare example of hydroxyl-directed C-H functionalisation has been reported by US chemists, demonstrating how molecular complexity can be drastically advanced in a single step.
Jin-Quan Yu and colleagues at the Scripps Research Institute, La Jolla, synthesised a series of 1-isochromanones – key structural motifs in natural products and drug candidates – using palladium-catalysed C-H carbonylation. The team found that amino acid ligands could promote the reaction, which is the first example of ligand-enabled C–H carbonylation.
The protocol represents a rare case in which the directing group (which typically needs to be removed after C-H functionalisation) and the coupling partner are fully incorporated into the target molecule without further synthetic manipulations. It is an encouraging step forward to improving atom- and step-economy in organic synthesis, says Yu.
Read Yu’s Edge article in Chemical Science to find out more.
Also of interest:
Professor Yu is guest editor of a recently published Chem Soc Rev themed issue on C-H functionalisation in organic synthesis. Read the issue today to stay abreast of this burgeoning field.