Dye sensitized solar cells (DSSCs) are photovoltaic devices which use a sensitizer in combination with a photosensitive material and a wide band gap semiconductor to produce an electric current. Thin-film DSSCs can offer packaging and application opportunites, have potential as functional coatings, and exhibit high power conversion efficiencies (PCE) of up to 12-13%.
Current state-of-the-art dye sensitized solar cells are usually solid phase systems, removing any issues surrounding leakage of components or the corrosive nature of some electrolytes. In such configurations a constituent known as a hole transport material (HTM) is now commonly used instead of a liquid electrolyte.
In this Chemical Science Edge article, authors from the Energy Research Institute, Nanyang, and the Nanyang Technological University, Singapore, give details of a group of triptycene based molecules which, they claim, perform on a comparable level with the current best in class HTM, (spiro-OMeTAD). The materials described were prepared from a starting tri-iodotryptycene material which was derivatised using a variety of transformations including Stille and Suzuki coupling reactions. The materials contain diphenylamine moieties separated from the triptycene core by aromatic spacers, enabling charge transfer.
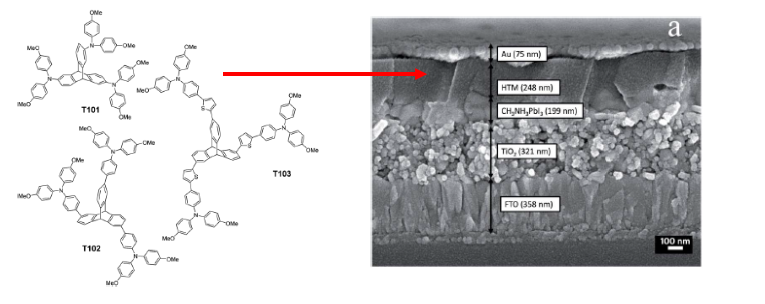
These materials were fabricated in a complex process into a typical DSSC, containing fluorine doped tin oxide, semiconducting titanium dioxide and lead iodide perovskite dye sensitizer material. Power conversion efficiencies are comparable to devices comprimising the best performing HTMs. For the T102 andT103 derivatives, PCEs were reported as 12.24 and 12.38%, comparable to devices fabricated with spiro-OMeTAD (12.78%). Without a HTM present the number falls to less than 5%.
This article presents details of promising HTM materials which may give rise to significant economic advantages for the production and commercialisation of solid state DSSCs, with current best in class performance levels.
Read this HOT Chemical Science Edge Article for free* today:
Novel hole transporting materials based on triptycene core for high efficiency mesoscopic perovskite solar cells
Anurag Krishna, Dharani Sabba, Hairong Li, Jun Yin, Pablo P. Boix, Cesare Soci, Subodh G. Mhaisalkar and Andrew C. Grimsdale
DOI: 10.1039/C4SC00814F

About the webwriter
Kevin Murnaghan is a guest web-writer for Chemical Communications. He is currently a Research Chemist in the Adhesive Technologies Business Sector of Henkel AG & Co. KGaA, based in Düsseldorf, Germany. His research interests focus primarily on enabling chemistries and technologies for next generation adhesives and surface treatments. Any views expressed here are his personal ones and not those of Henkel AG & Co. KGaA.
*Access is free untill the 11.07.14 through a registered RSC account – click here to register