The utility of palladium-catalyzed coupling has grown extensively as it has become one of the most powerful tools available to synthetic chemists. By playing a strategic role in the formation of carbon-carbon and carbon-heteroatom bonds, this synthetic technology has provided numerous opportunities to access diverse molecular architectures.
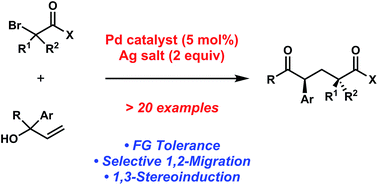
In this recently published Chemical Science Edge Article, Professor Uttam K. Tambar and colleague from the University of Texas Southwestern Medical Center at Dallas set out to determine whether the Mizoroki–Heck reaction could be extended to generate dicarbonyl species while circumventing the usual alkene products. They envisioned that under the modified reaction conditions the initial coupling of substituted allylic alcohols and organohalides would be followed by the key 1,2-migration step, instead of the conventional β-hydride elimination.
After screening various reaction conditions, the Tambar group found that the desired aryl dicarbonyls were furnished in good yield when a catalytic amount of bis(benzonitrile)dichloropalladium(II) was combined with a silver salt in the presence of 1,1-diphenylallyl alcohol and ethyl bromoacetate.
An investigation of the scope revealed that the aryl groups substituted with electron-rich and electron-deficient groups, as well as geminal-disubstituted allylic alcohols provided the desired coupling products. Based on the preliminary mechanistic studies, they proposed that the reactions proceeded through the acyclic free radical pathway.
Furthermore, Prof. Tambar and his coworker also tested the possibility of producing additional acyclic 1,5-dicarbonyl compounds containing multiple stereogenic centers. For instance, when they utilized the piperidine-substituted methyl bromoamide as the reaction partner with 1,1-diphenylallyl alcohol, the researchers found that the coupling reaction yielded the diketone product with diastereomeric ratio of 5:1.
Because acyclic stereocontrol remains a challenging research area in stereoselective free radical reactions, the 5:1 ratio is notably the highest reported level of 1,3-stereoinduction in 1,2-aryl migrations.
This exciting article was just published in Chemical Science as an Edge Article. Read “Palladium-catalyzed cross-coupling of α-bromocarbonyls and allylic alcohols for the synthesis of α-aryl dicarbonyl compounds” (DOI: 10.1039/C5SC00505A) by Professor Uttam K. Tambar and Yang Yu to learn more about their chemistry. The article is free to access until 20th May 2015*.
*Access is free through a registered RSC account
Dr. Tezcan Guney is a web writer for Chemical Society Reviews, Chemical Science and Chemical Communications. Dr. Guney received his Ph.D. from the Department of Chemistry at Iowa State University with Prof. George Kraus, where he focused on the synthesis of biologically active polycyclic natural products and multifunctional imaging probes. Currently, he is a postdoctoral research scholar at the Memorial Sloan-Kettering Cancer Center in New York with Prof. Derek Tan, contributing to the efforts to access biologically active small molecules using the diversity-oriented synthetic approach.
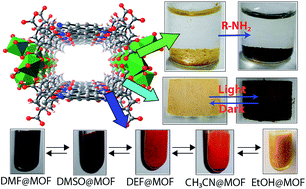
 Ionic liquid conductivity decreases at low temperature. To overcome this, a team of researchers from across Japan have combined an ionic liquid with a metal–organic framework (MOF) to produce an unusual material that retains its conductivity below –20°C. This low-temperature conductivity, together with the attractive ionic liquid properties of non-flammability and negligible volatility, could open up the potential for safe battery and capacitor materials for use in extremely cold conditions. Read the full article in Chemistry World»
Ionic liquid conductivity decreases at low temperature. To overcome this, a team of researchers from across Japan have combined an ionic liquid with a metal–organic framework (MOF) to produce an unusual material that retains its conductivity below –20°C. This low-temperature conductivity, together with the attractive ionic liquid properties of non-flammability and negligible volatility, could open up the potential for safe battery and capacitor materials for use in extremely cold conditions. Read the full article in Chemistry World»










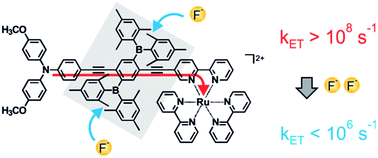
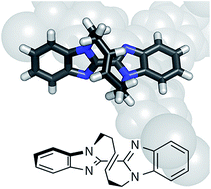
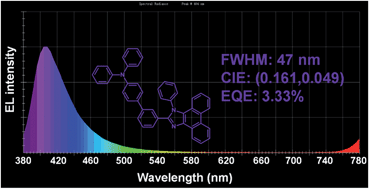

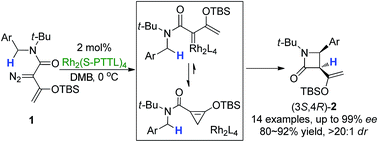

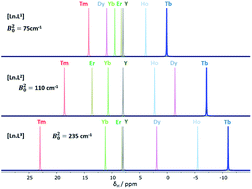
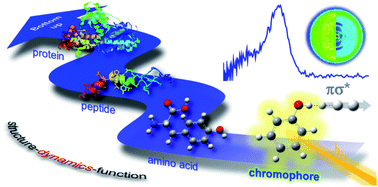
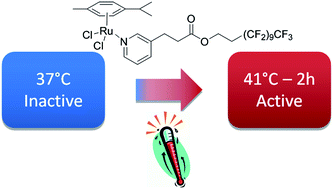
 Scientists in China have developed
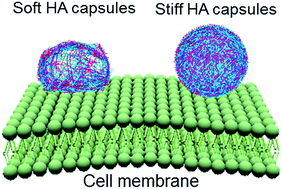
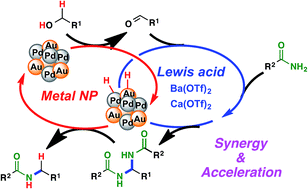
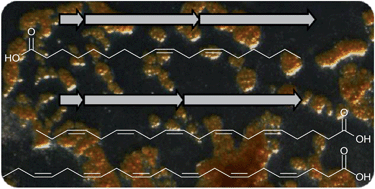
Scientists in China have developed  Inspired by nature, scientists in Australia have
Inspired by nature, scientists in Australia have