In this Chemical Science Edge article, Michael Ward, Christopher Hunter and co-workers at the Department of Chemistry, University of Sheffield report tunable host-guest chemistry of an octanuclear cobalt(II) coordination cage with a range of organic guests. The resultant non-covalently bound guests have very different binding constants in different pH environments, making binding tunable and completely reversible.
The team found that the coordination cage provided a good guest space for a range of organics, including substituted adamantanes. Substitutents which could be charged, such as carboxylic acids and amines, were particularly useful to drive and rationalize the binding observed. NMR also played a powerful role in this work. In this context, the paramagnetic complex host resulted in clear distinguishable signals, differentiating free or bound cage species, and thereby acting as a shift reagent. This effect could also be quantified, allowing the strength and extent of the host-guest binding to be shown.
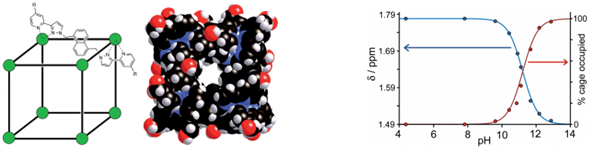
Coordination cage and NMR study of effect on chemical shift and binding extent with changing pH
An example with potentially high importance is described using 1-aminoadamantane, a prescription drug used to treat Parkinson’s disease and as an influenza anti-viral. Complete reversible binding was achieved with a binding constant difference of three orders of magnitude between the protonated and neutral forms of the drug. Similar behaviour was observed with other N-basic materials like nicotine and the anaesthetic substituted imidazole drug, demotidine. Mechanistic considerations were further examined with experiments using carboxylic acids, where solvation effects were also shown to play a key role.
In this article, a practical example of pH controlled, reversible guest binding of functional organic molecules is described. An elegant and practical application of NMR spectroscopy is also shown. Applications for delivery and controlled release of suitable drug, catalyst or other functional organic material cannot be far off.
Read this Chemical Science Edge article today!
pH dependent binding of guests in the cavity of a polyhedral coordination cage: reversible uptake and release of drug molecules
William Cullen, Simon Turega, Christopher A. Hunter and Michael D. Ward.,
doi 10.1039/c4sc02090a
 Flow reactors are edging towards self-regulation, thanks to researchers in the UK.
Flow reactors are edging towards self-regulation, thanks to researchers in the UK.












 University chemistry students are taught that the shapes and electronics of inorganic complexes are predictable. For example, d8 square-planar Pd(ii) and Pt(ii) complexes are invariably low spin, while d3–d7 tetrahedral complexes are high spin. Now, researchers in the US have thrown away the textbook by synthesising
University chemistry students are taught that the shapes and electronics of inorganic complexes are predictable. For example, d8 square-planar Pd(ii) and Pt(ii) complexes are invariably low spin, while d3–d7 tetrahedral complexes are high spin. Now, researchers in the US have thrown away the textbook by synthesising 


 Scientists in the US have developed
Scientists in the US have developed 