Glutathione is an antioxidant enzyme and the most abundant biothiol in human cells. It plays a crucial role in protecting cells against oxidative damage from various toxins that the body produces. However, abnormal levels of glutathione can lead to oxidative stress, which in turn can lead to premature aging and conditions such as Alzheimer’s or Parkinson’s disease.
There is, therefore, a need for the selective detection of glutathione, so that its role in biological systems can be better understood. However, it is challenging to design a selective fluorescent probe for a specific biothiol due to the structural and reactivity similarities with other biothiols. This is the challenge that David Churchill and team from the Department of Chemistry at the Korea Advanced Institute of Science and Technology set out to meet.
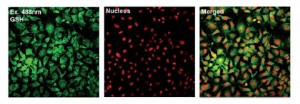
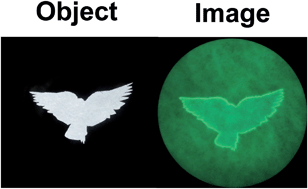
The team has previously explored the use of fluorescent probes containing selenium as the reactive centre and they have taken a similar approach with this challenge, using a phenylselenide group. The images below show the phenylselenide probe reacting with cellular glutathione, which fluoresces green in the images. The intensity of the fluorescence was around a hundred times greater than for cysteine or homocysteine, which are closely related to glutathione.
Until now, there has been no probe that can selectively detect glutathione in real time, so it will be interesting to see what future results come from this advance.
To read the details, download the Chemical Science article and read it in full – it’s open access:
Exceptional time response, stability and selectivity in doubly-activated phenyl selenium-based glutathione-selective platform
Youngsam Kim, Sandip V. Mulay, Minsuk Choi, Seungyoon B Yu, Sangyong Jon and David G Churchill
Chem. Sci., 2015, 6, Advance Article
DOI: 10.1039/C5SC02090E