Patrick Steel’s group at the University of Durham have used computational modelling in combination with experimental studies to generate silene equivalents.
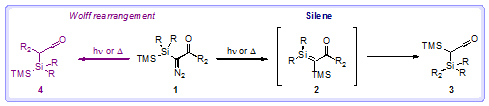
Silenes (2) are compounds containing a silicon–carbon double bond, which are generally so reactive that they are only transient in existence and rapidly rearrange to other products (3). Additionally, methods for the preparation of silenes are often incompatible with a range of functional groups. Photochemical or thermal decomposition of α-silyldiazocarbonyl compounds (1) is an established method for the formation of silenes (2); however, the Wolff rearrangement product (4) is also often formed under these conditions.
The Steel group aimed to generate a silene equivalent via a rhodium-catalysed decomposition of α-silyldiazocarbonyls. Computational studies enabled the group to determine that silene formation was favoured when electron-donating groups were attached to the carbonyl groups. With these results in hand, they synthesised α-hypersilyl diazoesters (5) and found that the resultant silene equivalents (6) were unusually stable and could also undergo reactions with α,β-unsaturated carbonyl compounds (7) to form cyclic silyl enol ethers (8). A subsequent fluoride-mediated fragmentation of the cycloadducts (8) gave 1,5-dicarbonyl products (9 and 10) in good yields.
The group successfully used results from their computational modelling to design more stable and synthetically useful silene equivalents. The mild reaction conditions developed represent an important advance in the use of silene reagents for organic synthesis.
“The general aim of my project was concerned with development of methods for the stereocontrolled functionalisation of alkenes through reaction with readily accessible silenes. I explored the synthetic potential of α-silyldiazocarbonyl compounds as silene precursors. I carried out the initial computational work at Uppsala University in Sweden under the supervision of Prof. Ottosson. These preliminary investigations identified targets, which I subsequently synthesised at Durham University. Interestingly, I found that α-silyl diazo esters undergo rhodium (II) catalysed decomposition to provide silaoxetene which shows silene-like reactivity. The silaoxetene is formed under very mild conditions, a fact which greatly improves its synthetic potential. I hope this will open the door for the use of silene equivalents in synthetic organic chemistry.” Michal Czyzewski, from the Steel group.
Find out more – download the Edge article for free.
Posted on behalf of Alice E. Williamson, Chemical Science web writer.