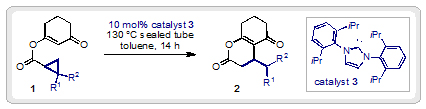
Researchers at Monash University in Australia have described the first example of a combined Brønsted/Lewis base cascade catalysis using an N-heterocyclic carbene (NHC) catalyst. David Lupton’s group have reported the synthesis of a variety of different dihydropyranones (2) in a single step from cyclopropyl enol esters (1).
Lisa Candish conducted mechanistic studies, which have enabled the group to suggest a possible catalytic cycle for the transformation. Firstly, catalytic deprotonation of 1a generates the α- or γ-enolate I which undergoes proton transfer to form the cyclopropyl anion II, primed for an electrocyclic ring opening to give allyl anion III. Protonation of III serves to regenerate catalyst 3, enabling its involvement in a second step of the mechanism. Addition of catalyst 3 to ester IV forms hemiacetal V, which undergoes a Claisen rearrangement to generate the final product 2a.
Exploitation of this dual Brønsted and Lewis base activation may enable the development of other interesting transformations. Additionally, the use of homochiral NHCs may enable enantioselective transformations in the future.
Lupton’s Chemical Science Edge article is free to download – read it today to find out more.
Determining a possible mechanism for this reaction was extremely challenging and enjoyable. A range of approaches were explored, but most lead to increased ambiguity. It was very satisfying when we came up with a strategy that actually helped clarify the reaction path. As a student this was very rewarding and reminded me of why I initially chose to study organic synthesis. Lisa Candish
Posted on behalf of Alice E. Williamson, Chemical Science web writer.