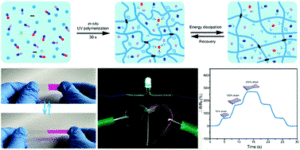
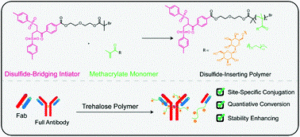
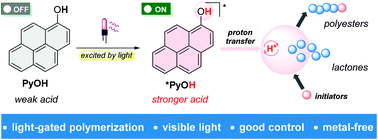
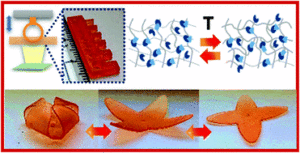
Ntetsikas et al. synthesised a series of linear and miktoarm star copolymers to study their self-assembly behaviour in bulk.
Block copolymers consisting of high 1,4-microstructure-content polybutadiene (PB1,4) blocks and high 3,4-content polyisoprene (PB1,4–b-PI3,4) blocks self-assemble, due to their incompatibility, to form different nanostructures useful for various applications, such as electronic devices, nanotechnology and optoelectronics. In this work, Avgeropoulos and co-workers report a new synthetic procedure for the preparation of four linear PB1,4–b-PI3,4 diblock copolymers and eight asymmetric miktoarm star copolymers and investigate the effect of the architecture (linear versus non-linear) on microphase separation and final nanostructure of these copolymers. Furthermore, the results of this study have been compared with the PS(PI1,4)n (PS: polystyrene) well studied and established systems.
In particular, the authors combined anionic polymerization and selective chlorosilane chemistry to prepare four different sets of linear and star copolymers. Each set included one linear diblock copolymer with similar molecular characteristics to the corresponding PB1,4(PI3,4)2 and PB1,4(PI3,4)3 miktoarm stars. All copolymers were carefully characterized by size-exclusion chromatography (SEC), membrane osmometry (MO) and nuclear magnetic resonance (NMR) indicating a high degree of molecular and compositional homogeneity. Differential scanning calorimetry (DSC) and transmission electron microscopy (TEM) were used to verify microphase separation and reveal the effect of the architecture on the adopted topologies. Through such a comprehensive characterization the authors have discovered that the high chain flexibility provided by the two polydiene segments affords promising properties previously unattainable from the corresponding triblock copolymers of these polydienes with polystyrene.
This work paves the way for further studies of material properties such as rheology and binary blends of the pure linear and non-linear copolymers with corresponding homopolymers (either hPB1,4 or hPI3,4).
We look forward to further exciting findings from the Avgeropoulos’ group.
Tips/comments directly from the authors:
- Morphological characterization studies reveal the coherence of theoretical studies on the PS(PI1,4)n system and the experimental results of the PB1,4(PI3,4)n system (PS is substituted by PI3,4).
- The only discrepancies from the relevant PS/PI system were found for two linear copolymers, where in both samples, hcp cylinders of the minority phase in the matrix of the majority were observed, instead of the expected DG cubic structure morphology.
- The almost identical electron densities between the two polydienes led to impossible morphological characterization through small angle X-ray scattering (SAXS) and only transmission electron microscopy results verify the adopted morphology for each copolymer.
- The adopted well-ordered nanostructures lead to the assumption that the segment–segment interaction parameter between the two polydienes of high 1,4-microstructure (∼92%) for the PB and ∼55–60% 3,4-microstructure for the PI is well above zero.
- It was really exciting to verify that if the 3,4-microstructure for the PI blocks was not within the regime of ∼55–60% then a homogeneous structure was adopted (no microphase separation).
- This regime of ∼55–60% 3,4-microstructure for the PI segments can be achieved by just adding a very small amount of a polar additive (∼1ml of THF) in the polymerization solvent ( 200 ml of benzene).
Citation to the paper: Synthesis, characterization and self-assembly of linear and miktoarm star copolymers of exclusively immiscible polydienes, Polym. Chem., 2021,12, 2712-2721, DOI: 10.1039/D1PY00258A
Link to the paper:
https://pubs.rsc.org/en/content/articlelanding/2021/py/d1py00258a#!divAbstract
 Dr. Athina Anastasaki is an Editorial Board Member and a Web Writer for Polymer Chemistry. Since January 2019, she joined the Materials Department of ETH Zurich as an Assistant Professor to establish her independent research group.
Dr. Athina Anastasaki is an Editorial Board Member and a Web Writer for Polymer Chemistry. Since January 2019, she joined the Materials Department of ETH Zurich as an Assistant Professor to establish her independent research group.