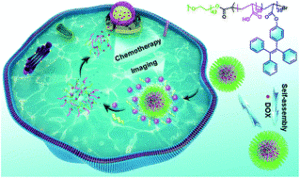
Yan et al. develop new enzyme-responsive polymeric micelles with potential applications in cancer therapy.
One of the most exciting and fast-growing topics in polymer chemistry is the synthesis of amphiphilic copolymers that can self-assemble into nanoparticles. Hydrophobic compounds such as cancer drugs can be encapsulated in the core of these self-assembled nanoparticles, thus protecting them from degradation or unwanted interactions with healthy cells. In addition, advances in polymer end-group functionalization allow the conjugation of special ligands on the nanoparticle surface which are responsible for directing the nanoparticles to cancer cells. Upon reaching the tumours (or being taken up by cancer cells), the nanoparticles must release the encapsulated drugs in order to kill the cancer cells. This drug release step requires the use of stimuli-responsive smart polymers that can switch from hydrophobic to hydrophilic upon exposure to stimuli. Temperature, pH, and enzyme-responsive polymers are therefore developed to release drugs on-demand. In this work, Zhao and co-workers further advance the field by synthesizing new fluorescent nanoparticles which can release a cancer drug (doxorubicin) while simultaneously turning off the fluorescent signal when the drug is released. This was achieved by efficiently coupling a tetraphenylethene moiety onto poly(acrylic acid). The hydrophobic property of the tetraphenylethene moiety induces the self-assembly of the resulting diblock copolymers into fluorescent nanoparticles via an aggregation-induced self-assembly mechanism. Upon exposure of the fluorescent nanoparticles to esterase, this enzyme can hydrolyze the ester bond between the tetraphenylethene side chain and the polymer backbone. The enzyme-catalyzed hydrolysis reaction turns the hydrophobic block back to the water-soluble poly(acrylic acid) block and therefore, disassembles the nanoparticles and also turns the fluorescent signal off. The diblock copolymer has poly(ethylene glycol) as the corona-forming block which possesses negligible toxicity to healthy cells. Therefore, this new copolymer is very promising for drug delivery applications, especially when monitoring the drug release is essential.
Citation to the paper: Visible light enabled para-fluoro-thiol ligation, Polym. Chem., 2020, 11, 7704-7713, DOI: 10.1039/D0PY01328E
Link to the paper: https://pubs.rsc.org/en/content/articlepdf/2020/py/d0py01328e
 Dr. Athina Anastasaki is an Editorial Board Member and a Web Writer for Polymer Chemistry. Since January 2019, she joined the Materials Department of ETH Zurich as an Assistant Professor to establish her independent research group.
Dr. Athina Anastasaki is an Editorial Board Member and a Web Writer for Polymer Chemistry. Since January 2019, she joined the Materials Department of ETH Zurich as an Assistant Professor to establish her independent research group.