Hans R. Kricheldorf studied chemistry at the University of Freiburg im Breisgau where he obtained his master degree in 1967 and his PhD in 1969. He continued his academic career as assistant Professor at the “Institut für Makromolekulare Chemie” in Freiburg im Breisgau and achieved the tenure (Habilitation) in 1975 (awarded a prize by the Association of the German Chemical Industry). He was appointed Associate Professor at the same institute in 1980 and Full Professor of polymer chemistry at the University of Hamburg in 1982. He retired in 2008, but continued to perform experimental research without coworkers. His life-long working fields were ring-opening polymerization and polycondensation. During the past twenty years his interest was focused on syntheses of biodegradable polymers, interactions between ROP and polycondensation, and syntheses of cyclic and multicyclic polymers. He is author or coauthor of more than 800 peer-reviewed papers and patents and author, coauthor and/or editor of a dozen of books. Recently he was awarded the “Korshak Prize” of the Russian Academy of Sciences for his life-work on polycondensation.
Hans R. Kricheldorf studied chemistry at the University of Freiburg im Breisgau where he obtained his master degree in 1967 and his PhD in 1969. He continued his academic career as assistant Professor at the “Institut für Makromolekulare Chemie” in Freiburg im Breisgau and achieved the tenure (Habilitation) in 1975 (awarded a prize by the Association of the German Chemical Industry). He was appointed Associate Professor at the same institute in 1980 and Full Professor of polymer chemistry at the University of Hamburg in 1982. He retired in 2008, but continued to perform experimental research without coworkers. His life-long working fields were ring-opening polymerization and polycondensation. During the past twenty years his interest was focused on syntheses of biodegradable polymers, interactions between ROP and polycondensation, and syntheses of cyclic and multicyclic polymers. He is author or coauthor of more than 800 peer-reviewed papers and patents and author, coauthor and/or editor of a dozen of books. Recently he was awarded the “Korshak Prize” of the Russian Academy of Sciences for his life-work on polycondensation.
What was your inspiration in becoming a polymer chemist?
My inspiration in becoming a polymer chemist had two sources. In the age of 15 I became interested in chemical experiments primarily in syntheses of explosives. In the age of 18 this hobby ended with a stay in a hospital, but at that time I was proud to have a collection of 27 different explosives. As a student I was mainly in organic chemistry, but when I had to select a research group for my PhD work, I decided for two reasons to enter the field of polymer chemistry. Firstly, I had the vision that there will be more space for fundamental research, because this part of chemistry was relatively new compared to organic and inorganic chemistry. Secondly, the university of Freiburg im Breisgau with the Institut für Makromolekulare Chemie was a particularly attractive place to start a career in polymer chemistry, because the first Nobel Prize laureate in polymer science, Prof. H. Staudinger, had worked here for more than 30 years. When I was a young student I saw him twice one year before his death and he impressed me. I joined the group of Assoc. Prof. G. Greber (later Full Prof. and director at the Tech. Univ. of Vienna) who was the last PhD student of H. Staudinger. By joining his group, I became so-to-say a scientific grandson of H. Staudinger.
What was the motivation behind your most recent Polymer Chemistry article ?
My motivation behind my recent work was an attempt to close two gaps in my knowledge about ring-opening polymerization of lactide (and lactones). For the technical production of poly(l-lactide) (meanwhile approaching 700 000 t/pa) tin(II) 2-ethlyhexanoate is the most widely used catalyst. For the technical production an alcohol is used as initiator to control the molecular weight and to accelerate the polymerization. However, over the past 20 years nobody has elucidated what happens in the absence of an initiator. Furthermore, I have recently defined and described a new type of polymerization called ROPPOC (ring-opening polymerization combined with simultaneous polycondensation). Introduction of a highly electrophilic and group via the catalyst is the key to success. However, catalysts introducing an anhydride group were still lacking in my collection of ROPPOC catalysts. The results obtained with Sn(II) acetate or 2-ethylhexanoate have now closed these gaps.
Which polymer scientist are you most inspired by?
My research activities of the past twenty years were mainly inspired by the work of Wallace H. Carothers, Paul J. Flory (Nobel Prize 1974) and Walter H. Stockmayer. These scientists formulated the experimental and theoretical fundament of step-growth polymerizations. However, when reading their papers, I had the impression that part of their theories, primarily their understanding of cyclization reactions, was incorrect. Therefore, I have spent much time and work with elaborating sufficient evidence for the correctness of my view. By the way, I became acquainted with both Flory and Stockmayer before 1985 and I was impressed by their personalities. But at that time, I had not worked yet on the aforementioned problems, so that our discussions touched other working fields.
How do you spend your spare time?
Forty years ago, I have begun to learn horse riding and over the past thirty years I had two horses. But recently I had to euthanize my second horse because of an accident, and now I’m to old to begin with a new horse. However, I continue to perform gym including bicycling and swimming to maintain my fitness as good as I can. Another major hobby is history, because any object and any idea has a history, and knowing more about the past means better understanding of the present. In this connection I have written several books after my retirement, for example a book about the most important 15 materials that form the fundament of our civilization, a book about history and philosophy of the natural sciences, a book about the history of polycondensation and most recently a book discussing the question, if life is the consequence of a chemical evolution.
What profession would you choose if you weren’t a scientist?
I had chosen to become physician, probably specialized in radiology. In the aftermath I indeed regret that I have decided to study chemistry. In the years 1995-2010 I had a cooperation with Prof. Ch. Jürgens (surgeon and director at a big hospital in Hamburg) on applications of biodegradable films for dressing of large burn wounds and as tissue-separating films. Our films (mainly consisting of lactide) were commercialized under the trade marks “Topkin” and “Mesofol “, but the Merck-Biomet company which produced these films stopped the production after ten years for financial reasons, and thus, our films did not become a big success. Nonetheless, our films supported an almost painless healing of more than 500 patients. From this cooperation I have learned that it is more satisfactory for me to help patients to recover from their wounds than publishing several more papers on polymer chemistry.
Read Hans’ full article now
High molar mass cyclic poly(l-lactide) obtained by means of neat tin(ii) 2-ethylhexanoate
Hans R. Kricheldorf and Steffen M. Weidner
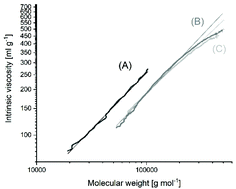
L-Lactide was polymerized in bulk at 120, 140, 160 and 180 °C with neat tin(II) 2-ethylhexanoate (SnOct2) as the catalyst. At 180 °C the Lac/Cat ratio was varied from 25/1 up to 8000/1 and at 160 °C from 25/1 up to 6000/1. The vast majority of the resulting polylactides consist of cycles in combination with a small fraction of linear chains having one octanoate and one COOH end group. The linear chains almost vanished at high Lac/Cat ratios, as evidenced by MALDI-TOF mass spectrometry and measurements of intrinsic viscosities and dn/dc values. At Lac/Cat ratios <1000/1 the number average molar masses (Mn) are far higher than expected for stoichiometic initiation, and above 400/1 the molar masses vary relatively little with the Lac/Cat ratio. At 180 °C slight discoloration even at short times and degradation of the molar masses were observed, but at 160 °C or below colorless products with weight average molar masses (Mw) up to 310 000 g mol−1 were obtained. The formation of high molar mass cyclic polylactides is explained by a ROPPOC (Ring-Opening Polymerizatiom with simultaneous Polycondensation) mechanism with intermediate formation of linear chains having one Sn–O–CH end group and one mixed anhydride end group. Additional experiments with tin(II)acetate as the catalyst confirm this interpretation. These findings together with the detection of several transesterification mechanisms confirm the previous critique of the Jacobson–Stockmayer theory.
About the Webwriter
 Simon Harrisson is a Chargé de Recherche at the Centre National de la Recherche Scientifique (CNRS), based at the Laboratoire de la Chimie des Polymères Organiques (LCPO) in Bordeaux, France. His research seeks to apply a fundamental understanding of polymerization kinetics and mechanisms to the development of new materials. He is an Advisory Board member for Polymer Chemistry. Follow him on Twitter @polyharrisson
Simon Harrisson is a Chargé de Recherche at the Centre National de la Recherche Scientifique (CNRS), based at the Laboratoire de la Chimie des Polymères Organiques (LCPO) in Bordeaux, France. His research seeks to apply a fundamental understanding of polymerization kinetics and mechanisms to the development of new materials. He is an Advisory Board member for Polymer Chemistry. Follow him on Twitter @polyharrisson