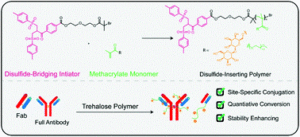
Forsythe and Maynard present a new strategy for the direct synthesis of disulphide-bridging trehalose polymers using a bis-sulfone ATRP initiator.
Conjugated polymers are often used to enhance the properties of therapeutic molecules such as antibodies and antigen binding fragments (Fabs). In this work, Forsythe and Maynard present a new strategy to prepare polymer-antibody/Fab conjugates by employing bis-sulfone end-groups installed via a functionalized atom transfer radical polymerization initiator. In particular, a bis-sulfone initiator was first synthesized and subsequently subjected to activators generated electron transfer (AGET) polymerization using ascorbic acid as the reducing agent. Upon optimizing the ligand concentration (special care was taken here as an excess of ligand causes a detrimental side reaction which results in broadening of the molecular weight distributions), disulphide-reactive trehalose polymers could be efficiently prepared with controlled molecular weight and fairly low dispersity, thus confirming a controlled polymerization. The polymers were then conjugated to a full Immunoglobulin G and its Fab fragment and the reaction proceeded quantitatively as confirmed by western blot and mass spectrometry. The stability of the resulting conjugates was then assessed by accelerated heat stress studies where the trehalose polymer was found to considerably increase the thermal stability of both Herceptin and Herceptin Fab. Importantly, this new strategy allows for a facile way to synthesize polymeric bioconjugates without the need for time consuming post-polymerization modification methods while also exhibiting very good monomer compatibility. As the authors conclude, they anticipate a continued exploration in the field of antibody and protein conjugation and we look forward to reading the next exciting findings from the Maynard group.
Tips/comments directly from the authors:
- The bis-sulfone functionality is a robust system for the production of protein-polymer conjugates. However, due to its base-sensitivity, care needs to be taken when incorporating it into polymers. Since common ligands for AGET ATRP display reactivity towards the bis-sulfone, both reaction temperature and concentration should be kept low.
- For performing conjugations using the bis-sulfone, we found it important to do a two-step reduction and alkylation where the reducing agent (DTT) was removed prior to the conjugation. To help avoid re-oxidation of the disulfides during this process, we used a buffer containing EDTA to prevent trace metal-mediated oxidation and additionally used desalting columns that allow for rapid removal of excess DTT.
- While we demonstrate the applicability of the bis-sulfone initiator for AGET ATRP, the chemistry should be amenable to other controlled polymerizations such as RAFT. Incorporation into a chain transfer agent could further expand the diversity of chemistry available for antibody conjugation.
- Trehalose polymers stabilized the antibody and Fab to temperature increases. However, the same polymer could also increase the stability of the conjugates in vivo since these polymers have improved the pharmacokinetics of other proteins.
Citation to the paper: Synthesis of disulfide-bridging trehalose polymers for antibody and Fab conjugation using a bis-sulfone ATRP initiator, Polym. Chem., 2021,12, 1217-1223, DOI: 10.1039/D0PY01579B
Link to the paper:
https://pubs.rsc.org/en/content/articlepdf/2021/py/d0py01579b
 Dr. Athina Anastasaki is an Editorial Board Member and a Web Writer for Polymer Chemistry. Since January 2019, she joined the Materials Department of ETH Zurich as an Assistant Professor to establish her independent research group.
Dr. Athina Anastasaki is an Editorial Board Member and a Web Writer for Polymer Chemistry. Since January 2019, she joined the Materials Department of ETH Zurich as an Assistant Professor to establish her independent research group.