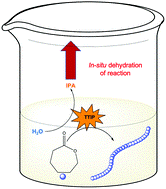
Atta et al. demonstrates a simplified ROP protocol which operates in the absence of any inert gas and without the need of drying any of the reaction’s reagents.
Ring Opening Polymerization (ROP) is arguably one of the most popular methodologies to synthesize biodegradable materials such as polycaprolactone (PCL) and poly (lactic acid) (PLA). However, a major drawback of this approach which severely limits its applicability is that it typically operates under completely moisture-free conditions, as water is well-known to deactivate the catalyst and terminate the propagating chains. To avoid water contamination, highly specialized equipment (e.g., Schlenk lines or glove boxes) as well as anhydrous reagents have to be employed which makes the process particularly tedious for both experts and non-experts. To overcome this, Gormley and co-workers have developed two elegant and simple methods that allow for the facile synthesis of PCL through ROP in a laboratory oven and without using any inert gas or dry reagents. In the first technique, a vacuum oven was employed to evaporate water from a traditional ROP reaction with stannous octoate as the catalyst while in the second approach titanium isopropoxide was utilized to simultaneously quench residual water and catalyze ROP. Impressively, and despite the simplicity of those methodologies, a range of chain lengths could be synthesized (degree of polymerization 25-500) with relatively good control over the molecular weight distributions of PCL (Đ < 1.5 for all cases). It is highlighted that a large excess of water impurities (750 ppm) could be tolerated by both methods yielding well-defined polymers at quantitative conversions. This work represents a great example of a simplified ROP which operates in the absence of complicated reactions set ups and can be performed in any laboratory. As the authors also remark, targeting even higher molecular weights or achieving even lower dispersity values will be the next challenge to address and we very much look forward to the next developments by the Gormley group.
Tips/comments directly from the authors:
- The rational goal of this work is to enable the ROP reaction in an oven without inert gas environment and without drying or purifying the reagents.
- The most exciting aspect of this work is to enable non-experts to synthesize custom polymers.
- TTIP plays multiple roles in this ROP reaction. It not only initiates and catalyzes the polymerization reaction but also eliminates water from the reaction medium.
- It is important for the audience that we should perform this experiment with minimal mixing time (within 1-5 sec) as water present in the air can contaminate CL.
- The purity of CL can be easily checked by TTIP. A precipitate of TiO2 was formed when the water content of CL was above 750 ppm, and a cloudy solution was observed.
Citation to the paper: Ring opening polymerization of ε-caprolactone through water, Polym. Chem., 2021,12, 159-164, DOI: 10.1039/D0PY01481H
Link to the paper: https://pubs.rsc.org/en/content/articlepdf/2021/py/d0py01481h
 Dr. Athina Anastasaki is an Editorial Board Member and a Web Writer for Polymer Chemistry. Since January 2019, she joined the Materials Department of ETH Zurich as an Assistant Professor to establish her independent research group.
Dr. Athina Anastasaki is an Editorial Board Member and a Web Writer for Polymer Chemistry. Since January 2019, she joined the Materials Department of ETH Zurich as an Assistant Professor to establish her independent research group.