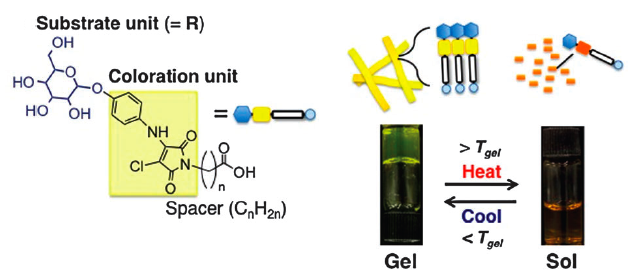
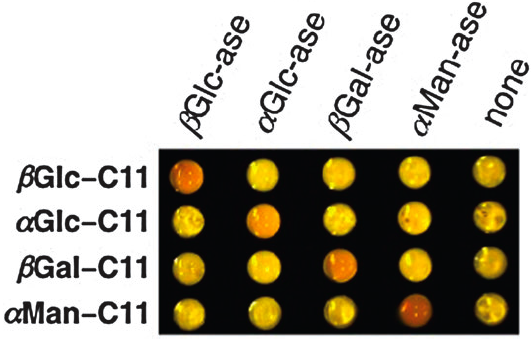
Scientists, led by Renato Zenobi of the Swiss Federal Institute of Technology (ETH) in Zurich, have been investigating metabolites in exhaled breath, showing that each person’s breath holds a unique, characteristic molecular ‘breathprint,’ as recently featured on the BBC website. This means that high-precision chemical analysis of a patient’s breath can potentially provide an instant, pain-free and non-invasive medical diagnosis, and may even provide an early warning for healthy persons at risk for certain diseases. In the future, it may also be used to calculate safe dosages of anaesthesia tailored to each patient’s metabolism and tolerance, or as a fast and convenient doping check for athletes.
Using mass spectrometry, Zenobi and his team regularly measured and analysed the exhaled breath of eleven volunteers for eleven days, finding that each individual’s metabolic ‘breathprint’ showed a unique core pattern and remained stable enough to be useful for medical purposes. Their mass spectra of exhaled breath have shown peaks or signals representing around a hundred compounds, most of which they are just beginning to identify and assign.
Their findings represent a significant step towards ‘personalised medicine,’ and show great potential for other applications, such as in forensics or metabolomics.
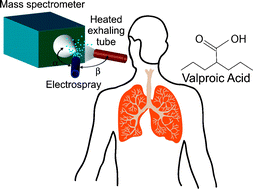
Zenobi and his co-workers first published their early work in chemical breath analysis in a 2011 ChemComm article, in which they used their novel method to identify valproic acid, a medication for epilepsy, in exhaled breath.
Read the ChemComm article where it all began!
Real-time, in vivo monitoring and pharmacokinetics of valproic acid via a novel biomarker in exhaled breath
Gerardo Gamez, Liang Zhu, Andreas Disko, Huanwen Chen, Vladimir Azov, Konstantin Chingin, Günter Krämer and Renato Zenobi
Chem. Commun., 2011, 47, 4884-4886
DOI: 10.1039/C1CC10343A












![Pillar[5]arene structure](https://blogs.rsc.org/cc/files/2013/03/Pillar5arene_Feb2013-300x239.png)
 Read this ChemComm cover article today:
Read this ChemComm cover article today: