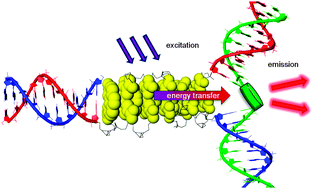
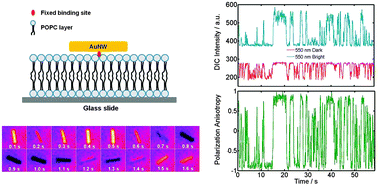
A modular LHC built on the DNA three-way junction
Markus Probst, Simon M. Langenegger and Robert Häner
Chem. Commun., 2014, 50, 159-161
DOI: 10.1039/C3CC47490A, Communication
Free to access until 19th January 2014
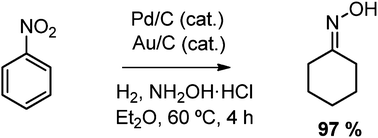
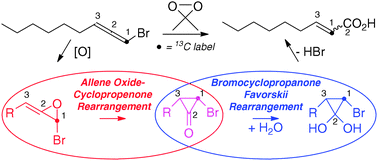
One pot synthesis of cyclohexanone oxime from nitrobenzene using a bifunctional catalyst
Paula Rubio-Marqués, Juan Carlos Hernández-Garrido, Antonio Leyva-Pérez and Avelino Corma
Chem. Commun., 2014, Advance Article
DOI: 10.1039/C3CC47693F, Communication
Free to access until 19th January 2014
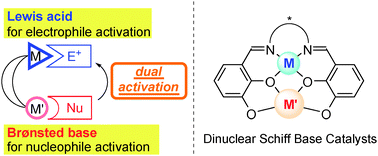
Recent advances in cooperative bimetallic asymmetric catalysis: dinuclear Schiff base complexes
Shigeki Matsunaga and Masakatsu Shibasaki
Chem. Commun., 2014, Advance Article
DOI: 10.1039/C3CC47587E, Feature Article
Free to access until 19th January 2014
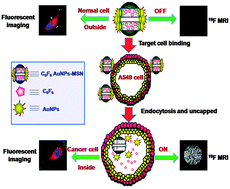
From assembled metal–organic framework nanoparticles to hierarchically porous carbon for electrochemical energy storage
Arlin Jose Amali, Jian-Ke Sun and Qiang Xu
Chem. Commun., 2014, Advance Article
DOI: 10.1039/C3CC48112C, Communication
Free to access until 19th January 2014
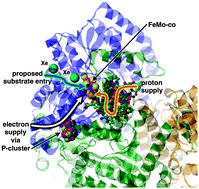
Nitrogenase: a general hydrogenator of small molecules
Ian Dance
Chem. Commun., 2013, 49, 10893-10907
DOI: 10.1039/C3CC46864J, Feature Article
Free to access until 19th January 2014
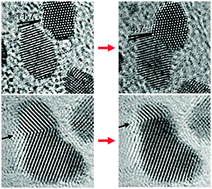
In situ atomic imaging of coalescence of Au nanoparticles on graphene: rotation and grain boundary migration
Jong Min Yuk, Myoungho Jeong, Sang Yun Kim, Hyeon Kook Seo, Jihyun Kim and Jeong Yong Lee
Chem. Commun., 2013, 49, 11479-11481
DOI: 10.1039/C3CC46545D, Communication
From themed collection Structure and chemistry of materials from in-situ electron microscopy
Free to access until 19th January 2014
THAT’S NOT ALL! Click here for more free HOT ChemComm articles for December!!