We are delighted to share with you the top 25 most downloaded articles in ChemComm from April–June 2014.
 Top 25 most downloaded ChemComm articles for Q2 2014
Top 25 most downloaded ChemComm articles for Q2 2014
Nanoparticles act as protein carriers during cellular internalization
Gerard W. Doorley and Christine K. Payne
DOI: 10.1039/C2CC16937A
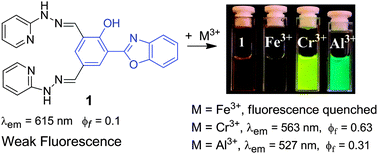
Alzheimer’s disease amyloid beta converting left-handed Z-DNA back to right-handed B-form
Jie Geng, Chuanqi Zhao, Jinsong Ren and Xiaogang Qu
DOI: 10.1039/C0CC02049D
Polyfunctional benzylic zinc chlorides by the direct insertion of magnesium into benzylic chlorides in the presence of LiCl and ZnCl2
Albrecht Metzger, Fabian M. Piller and Paul Knochel
DOI: 10.1039/B812396A
Proton-regulated rectified ionic transport through solid-state conical nanopores modified with phosphate-bearing polymer brushes
Basit Yameen, Mubarak Ali, Reinhard Neumann, Wolfgang Ensinger, Wolfgang Knoll and Omar Azzaroni
DOI: 10.1039/B920870D
Wet chemical synthesis of silver nanorods and nanowires of controllable aspect ratio
Nikhil R. Jana, Latha Gearheart and Catherine J. Murphy
DOI: 10.1039/B100521I
Self-assembly of functional columnar liquid crystals
Takashi Kato, Takuma Yasuda, Yuko Kamikawa and Masafumi Yoshio
DOI: 10.1039/B816624B
Multifunctional catalysis by Pd-polyoxometalate: one-step conversion of acetone to methyl isobutyl ketone
Robert D. Hetterley, Elena F. Kozhevnikova and Ivan V. Kozhevnikov
DOI: 10.1039/B515325E
A robust, catalytic metal–organic framework with open 2,2′-bipyridine sites
Honghan Fei and Seth M. Cohen
DOI: 10.1039/C4CC01607F
Examination of native chemical ligation using peptidyl prolyl thioesters
Takahiro Nakamura, Akira Shigenaga, Kohei Sato, Yusuke Tsuda, Ken Sakamoto and Akira Otaka
DOI: 10.1039/C3CC47228K
Photo- and electro-chromism of diarylethene modified ITO electrodes—towards molecular based read–write–erase information storage
Jetsuda Areephong, Wesley R. Browne, Nathalie Katsonis and Ben L. Feringa
DOI: 10.1039/B608502D
Aggregation-induced emission: phenomenon, mechanism and applications
Yuning Hong, Jacky W. Y. Lam and Ben Zhong Tang
DOI: 10.1039/B904665H
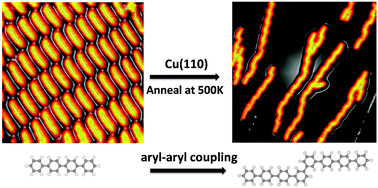
Direct arylation of pyridines without the use of a transition metal catalyst
Yahui Li, Wei Liu and Chunxiang Kuang
DOI: 10.1039/C4CC02546F
One-pot synthesis of magnetic particle-embedded porous carbon composites from metal–organic frameworks and their sorption properties
Hee Jung Lee, Won Cho, Eunji Lim and Moonhyun Oh
DOI: 10.1039/C4CC01914H
Interaction of modified liposomes with Bacillus spores
Sergey Kazakov, Marian Kaholek, Tao Ji, Charles L. Turnbough, Jr and Kalle Levon
DOI: 10.1039/B312389H
A facile one-pot method to high-quality Ag-graphene composite nanosheets for efficient surface-enhanced Raman scattering
Zhe Zhang, Fugang Xu, Wenshu Yang, Mingyi Guo, Xiaodan Wang, Bailin Zhang and Jilin Tang
DOI: 10.1039/C1CC11125F
Graphene quantum dots: emergent nanolights for bioimaging, sensors, catalysis and photovoltaic devices
Jianhua Shen, Yihua Zhu, Xiaoling Yang and Chunzhong Li
DOI: 10.1039/C2CC00110A
Nitrogen-doped carbon nanotubes and graphene composite structures for energy and catalytic applications
Won Jun Lee, Uday Narayan Maiti, Ju Min Lee, Joonwon Lim, Tae Hee Han and Sang Ouk Kim
DOI: 10.1039/C4CC00146J
Novel hole transporting materials with a linear π-conjugated structure for highly efficient perovskite solar cells
Junjie Wang, Shirong Wang, Xianggao Li, Lifeng Zhu, Qingbo Meng, Yin Xiao and Dongmei Li
DOI: 10.1039/C4CC01637H
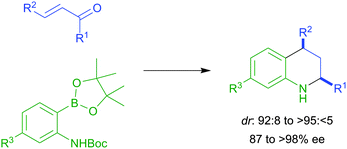
Metal-free oxidative synthesis of quinazolinones via dual amination of sp3 C–H bonds
Dan Zhao, Teng Wang and Jian-Xin Li
DOI: 10.1039/C4CC02648A
A dual functional additive for the HTM layer in perovskite solar cells
Hong Zhang, Yantao Shi, Feng Yan, Liang Wang, Kai Wang, Yujin Xing, Qingshun Dong and Tingli Ma
DOI: 10.1039/C3CC49458F
Copper-catalyzed olefinic C–H difluoroacetylation of enamides
Gilles Caillot, Jérémy Dufour, Marie-Charlotte Belhomme, Thomas Poisson, Laurence Grimaud, Xavier Pannecoucke and Isabelle Gillaizeau
DOI: 10.1039/C4CC01994F
Room-temperature Cu(II)-catalyzed aromatic C–H azidation for the synthesis of ortho-azido anilines with excellent regioselectivity
Yunpeng Fan, Wen Wan, Guobin Ma, Wei Gao, Haizhen Jiang, Shizheng Zhu and Jian Hao
DOI: 10.1039/C4CC01481B
Metal-mediated transformations of small molecules
Louise A. Berben and Jason B. Love
DOI: 10.1039/C4CC90123A
From themed collection Metal-Mediated Transformations of Small Molecules
A highly efficient mesoscopic solar cell based on CH3NH3PbI3−xClx fabricated via sequential solution deposition
Yingzhuang Ma, Lingling Zheng, Yao-Hsien Chung, Saisai Chu, Lixin Xiao, Zhijian Chen, Shufeng Wang, Bo Qu, Qihuang Gong, Zhaoxin Wu and Xun Hou
DOI: 10.1039/C4CC01962H
Reduction of graphene oxide viaL-ascorbic acid
Jiali Zhang, Haijun Yang, Guangxia Shen, Ping Cheng, Jingyan Zhang and Shouwu Guo
DOI: 10.1039/B917705A
ChemComm is the home of urgent high quality communications from across the chemical sciences. With a world renowned reputation for quality and fast times to publication (average of 40 days), ChemComm is the ideal place to publish your research.
Submit your urgent research to ChemComm today!
Stay up to date with ChemComm
Be among the first to hear about the newest articles being published – Sign-up to our journal news alert to receive information about most read articles, themed issues, journal news, as well as calls for papers and invitations.
Comments Off on Top 25 ChemComm articles April–June 2014



















 Covalency, a term describing bonding by sharing electrons, divides opinion when mentioned alongside hydrogen bonding. Worried that the concept of hydrogen bonding has been getting fuzzier over time, scientists in Germany have sought
Covalency, a term describing bonding by sharing electrons, divides opinion when mentioned alongside hydrogen bonding. Worried that the concept of hydrogen bonding has been getting fuzzier over time, scientists in Germany have sought  Top 25 most downloaded ChemComm articles for Q2 2014
Top 25 most downloaded ChemComm articles for Q2 2014