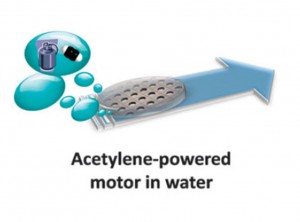
We are delighted to share with you the top 25 most downloaded articles in Chemical Communications (ChemComm) from July–September 2014.
Alzheimer’s disease amyloid beta converting left-handed Z-DNA back to right-handed B-form
Jie Geng, Chuanqi Zhao, Jinsong Ren and Xiaogang Qu
DOI: 10.1039/C0CC02049D, Communication
Fabricating graphene supercapacitors: highlighting the impact of surfactants and moieties
Dale A. C. Brownson and Craig E. Banks
DOI: 10.1039/C1CC11276G, Communication
From themed collection Emerging Investigators 2012
Two-dimensional layered composite photocatalysts
Jingxiang Low, Shaowen Cao, Jiaguo Yu and Swelm Wageh
DOI: 10.1039/C4CC02553A, Feature Article
Highly plasmon-enhanced upconversion emissions from Au@β-NaYF4:Yb,Tm hybrid nanostructures
Ning Liu, Weiping Qin, Guanshi Qin, Tao Jiang and Dan Zhao
DOI: 10.1039/C1CC11179E, Communication
A hydrophobic hole transporting oligothiophene for planar perovskite solar cells with improved stability
Lingling Zheng, Yao-Hsien Chung, Yingzhuang Ma, Lipei Zhang, Lixin Xiao, Zhijian Chen, Shufeng Wang, Bo Qu and Qihuang Gong
DOI: 10.1039/C4CC04680C, Communication
A rapid synthesis of high aspect ratio copper nanowires for high-performance transparent conducting films
Shengrong Ye, Aaron R. Rathmell, Ian E. Stewart, Yoon-Cheol Ha, Adria R. Wilson, Zuofeng Chen and Benjamin J. Wiley
DOI: 10.1039/C3CC48561G, Communication
Star-shaped hole transporting materials with a triazine unit for efficient perovskite solar cells
Kwangseok Do, Hyeju Choi, Kimin Lim, Hyunjun Jo, Jin Woo Cho, Mohammad K. Nazeeruddin and Jaejung Ko
DOI: 10.1039/C4CC04550E, Communication
Incorporation of iron hydrogenase active sites into a highly stable metal–organic framework for photocatalytic hydrogen generation
Koroush Sasan, Qipu Lin, ChengYu Mao and Pingyun Feng
DOI: 10.1039/C4CC03946G, Communication
Electrochemical activation of carbon dioxide in ionic liquid: synthesis of cyclic carbonates at mild reaction conditions
Hongzhou Yang, Yanlong Gu, Youquan Deng and Feng Shi
DOI: 10.1039/B108451H, Communication
A quick, simple, robust method to measure the acidity of ionic liquids
John Gräsvik, Jason P. Hallett, Trang Quynh To and Tom Welton
DOI: 10.1039/C4CC02816C, Communication
Modulating DNA-templated silver nanoclusters for fluorescence turn-on detection of thiol compounds
Zhengzhen Huang, Fang Pu, Youhui Lin, Jinsong Ren and Xiaogang Qu
DOI: 10.1039/C0CC05651K, Communication
CH3NH3PbI(3−x)(BF4)x: molecular ion substituted hybrid perovskite
Satyawan Nagane, Umesh Bansode, Onkar Game, Shraddha Chhatre and Satishchandra Ogale
DOI: 10.1039/C4CC04537H, Communication
Fluorescent probes for hydrogen sulfide detection and bioimaging
Fabiao Yu, Xiaoyue Han and Lingxin Chen
DOI: 10.1039/C4CC03312D, Feature Article
Synthesis of 1,2-amino alcohols via catalytic C–H amidation of sp3 methyl C–H bonds
Taek Kang, Heejeong Kim, Jeung Gon Kim and Sukbok Chang
DOI: 10.1039/C4CC05655H, Communication
Synthesis and characterization of multi-helical DNA–silica fibers
Yuanyuan Cao, Junjie Xie, Ben Liu, Lu Han and Shunai Che
DOI: 10.1039/C2CC37470F, Communication
Recent advances in the catalytic asymmetric 1,3-dipolar cycloaddition of azomethine ylides
Javier Adrio and Juan C. Carretero
DOI: 10.1039/C4CC04381B, Feature Article
Wet chemical synthesis of silver nanorods and nanowires of controllable aspect ratio
Nikhil R. Jana, Latha Gearheart and Catherine J. Murphy
DOI: 10.1039/B100521I, Communication
A metal free domino synthesis of 3-aroylindoles via two sp3 C–H activation
Anupal Gogoi, Anju Modi, Srimanta Guin, Saroj Kumar Rout, Debapratim Das and Bhisma K. Patel
DOI: 10.1039/C4CC04407J, Communication
Self-assembly of supramolecularly engineered polymers and their biomedical applications
Dali Wang, Gangsheng Tong, Ruijiao Dong, Yongfeng Zhou, Jian Shen and Xinyuan Zhu
DOI: 10.1039/C4CC03155E, Feature Article
From themed collection Polymer Self-Assembly
Nitrogen-centered radical-mediated C–H imidation of arenes and heteroarenes via visible light induced photocatalysis
Hyejin Kim, Taehoon Kim, Dong Gil Lee, Sang Weon Roh and Chulbom Lee
DOI: 10.1039/C4CC03905J, Communication
Visible light-promoted metal-free sp3-C–H fluorination
Ji-Bao Xia, Chen Zhu and Chuo Chen
DOI: 10.1039/C4CC05650G, Communication
Cu-Catalyzed Suzuki–Miyaura reactions of primary and secondary benzyl halides with arylboronates
Yan-Yan Sun, Jun Yi, Xi Lu, Zhen-Qi Zhang, Bin Xiao and Yao Fu
DOI: 10.1039/C4CC05376A, Communication
Nanostructured electrochromic smart windows: traditional materials and NIR-selective plasmonic nanocrystals
Evan L. Runnerstrom, Anna Llordés, Sebastien D. Lounis and Delia J. Milliron
DOI: 10.1039/C4CC03109A, Feature Article
Are Zr6-based MOFs water stable? Linker hydrolysis vs. capillary-force-driven channel collapse
Joseph E. Mondloch, Michael J. Katz, Nora Planas, David Semrouni, Laura Gagliardi, Joseph T. Hupp and Omar K. Farha
DOI: 10.1039/C4CC02401J, Communication
Graphene quantum dots: emergent nanolights for bioimaging, sensors, catalysis and photovoltaic devices
Jianhua Shen, Yihua Zhu, Xiaoling Yang and Chunzhong Li
DOI: 10.1039/C2CC00110A, Feature Article
ChemComm is the home of urgent high quality communications from across the chemical sciences. With a world renowned reputation for quality and fast times to publication (average of 40 days), ChemComm is the ideal place to publish your research.
Submit your urgent research to ChemComm today!
Comments Off on Top 25 ChemComm articles for July–September 2014
 Resembling a bundle of cereal stalks, this atomic force microscopy (AFM) image depicts the first ionic organic nanocrystals to have a sheaf-like structure. Ursula Mazur, of Washington State University, US, and colleagues grew the π-conjugated molecules from two electroactive porphyrin synthons: tetra(4-aminophenyl)porphyrin and tetra(4-sulfonatophenyl)porphyrin. These building blocks assembled themselves into branched structures – the higher the reaction temperature the longer and more numerous their branches.
Resembling a bundle of cereal stalks, this atomic force microscopy (AFM) image depicts the first ionic organic nanocrystals to have a sheaf-like structure. Ursula Mazur, of Washington State University, US, and colleagues grew the π-conjugated molecules from two electroactive porphyrin synthons: tetra(4-aminophenyl)porphyrin and tetra(4-sulfonatophenyl)porphyrin. These building blocks assembled themselves into branched structures – the higher the reaction temperature the longer and more numerous their branches.











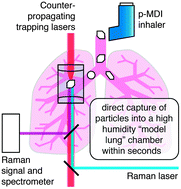
 The
The 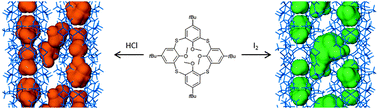
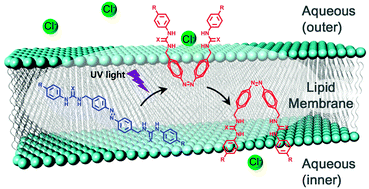
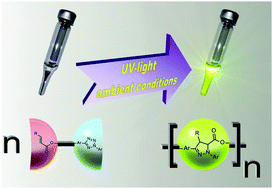

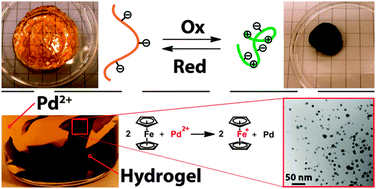

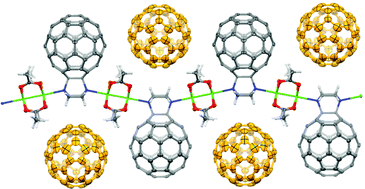
 Anthea Blackburn is a guest web writer for Chemical Communications.
Anthea Blackburn is a guest web writer for Chemical Communications.