Graphene, a two-dimensional single-layer graphite sheet, has aroused worldwide attention since the last decade. Its ultrahigh electrical and thermal conductivities, high mechanical stiffness and unique band structure have attracted extensive research efforts to develop graphene-based electronics, photonics, printing materials etc. Currently, among various strategies, the wet-chemical method still remains the most practical protocol for large-scale production of graphene in laboratories. This process in general involves two steps: the oxidative exfoliation of graphite, a.k.a. Hummers’ method, followed by reduction of the oxidized graphite sheets. Graphite oxide (GO), possessing a layered structure analogous to graphite but with rich oxygen functionalities (such as hydroxyl and carboxyl groups) anchored on each layer, is the product of the first step and thus serves as a precursor of graphene.
As the aforementioned wet chemical method is usually carried out in water, GO is primarily stored as aqueous-based colloidal dispersions. However, GO is reported to be chemically unstable in water since water molecules can react with electropositive carbons of GO. Though the reaction is not rapid, it partially removes the oxygen functionalities and breaks the carbon matrix, which eventually forces GO to precipitate and reduces the shelf life of the GO precursor.
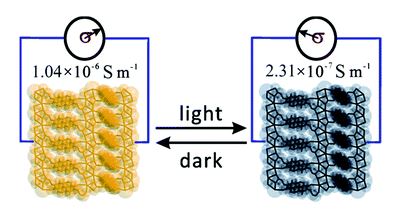
Recently, Shi and coworkers from Tsinghua University have successfully prolonged the lifetime of GO by dispersing it in organic solvents. During the last purification step of the Hummers’ method, instead of using de-ionized water, anhydrous ethanol was utilized to rinse the GO product and obtain ethanol-wetted GO. X-ray diffraction revealed that ethanol molecules existed in the inter-layer space between adjacent layers. The ethanol-wetted GO could be readily dissolved in propylene carbonate, an organic solvent, for concentrations ranging from 0.1 mg mL-1 to 40 mg mL-1 (Figures a and b). More importantly, GO could be stored in propylene carbonate for at least a month without a colour change, whilst the colour of aqueous GO dispersion discernibly darkened (Figure c). Spectroscopic studies indicated that the colour change was attributed to the loss of oxygen functionalities. The results unambiguously prove that GO in propylene carbonate is much more stable than GO in water.

Figure. (a) Dissolution of ethanol-wetted GO in propylene carbonate. (b) GO colloidal dispersions with various concentrations. (c) Color evolution of GO dispersions (1 mg mL-1) with water and propylene carbonate as solvents before and after storing for 28 days under ambient conditions.
Aside from propylene carbonate, dimethyl sulfoxide, ethylene glycol and N,N-dimethylformamide are solvents that can dissolve the ethanol-wetted GO. The successful stabilization of GO colloidal dispersions could ensure the steady production of graphene in laboratories, as well as reveal new opportunities to develop GO-based devices.
To find out more please read:
Organic Dispersions of Graphene Oxide with Arbitrary Concentrations and Improved Chemical Stability
Wencheng Du, Mingmao Wu, Miao Zhang, Guochuang Xu, Tiantian Gao, Liu Qian, Xiaowen Yu, Fengyao Chi, Chun Li and Gaoquan Shi
DOI: 10.1039/c7cc04584k
About the author:

Tianyu Liu is a Ph.D. in chemistry graduated from University of California-Santa Cruz. He is passionate about scientific communication to introduce cutting-edge researches to both the general public and the scientists with diverse research expertise. He is a web blog writer for the Chem. Commun. and Chem. Sci. blog websites. More information about him can be found at http://liutianyuresearch.weebly.com/.
Comments Off on Dissolving and Stabilizing the Precursor of Graphene in Organic Solvents















.jpg)
 Tianyu Liu is a Ph.D. in chemistry graduated from University of California-Santa Cruz. He is passionate about scientific communication to introduce cutting-edge researches to both the general public and the scientists with diverse research expertise. He is a web writer for the Chem. Commun. and Chem. Sci. blog websites. More information about him can be found
Tianyu Liu is a Ph.D. in chemistry graduated from University of California-Santa Cruz. He is passionate about scientific communication to introduce cutting-edge researches to both the general public and the scientists with diverse research expertise. He is a web writer for the Chem. Commun. and Chem. Sci. blog websites. More information about him can be found