All of the referee-recommended articles below are free to access until 6th July 2018.
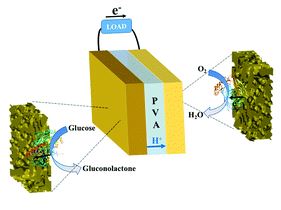
A quasi-solid-state and self-powered biosupercapacitor based on flexible nanoporous gold electrodes
Xinxin Xiao and Edmond Magner
Chem. Commun., 2018, 54, 5823-5826
DOI: 10.1039/C8CC02555J, Communication
_______________________________________________________________
Late stage modifications of P-containing ligands using transition-metal-catalysed C–H bond functionalisation
Zhuan Zhang, Pierre H. Dixneuf and Jean-François Soulé
Chem. Commun., 2018, Advance Article
DOI: 10.1039/C8CC02821D, Feature Article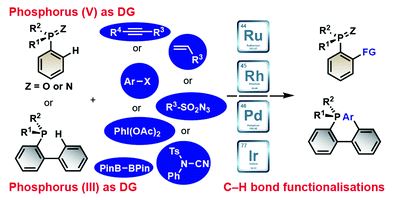
_______________________________________________________________
A novel three-fluorophore system as a ratiometric sensor for multiple protease detection
Yana Okorochenkova, Martin Porubský, Sandra Benická and Jan Hlaváč
Chem. Commun., 2018, Advance Article
DOI: 10.1039/C8CC01731J, Communication
_______________________________________________________________
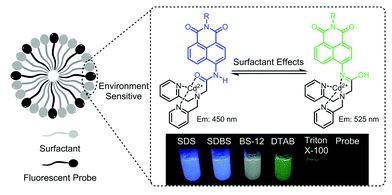
The environmental-sensitivity of a fluorescent ZTRS–Cd(II) complex was applied to discriminate different types of surfactants and determine their CMC values
Fei Deng, Shuangshuang Long, Qinglong Qiao and Zhaochao Xu
Chem. Commun., 2018, Advance Article
DOI: 10.1039/C8CC03888K, Communication
_______________________________________________________________
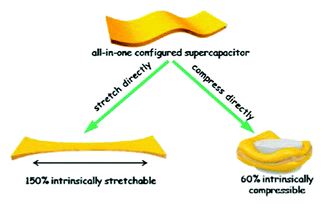
An intrinsically compressible and stretchable all-in-one configured supercapacitor
Mengmeng Hu, Jiaqi Wang, Jie Liu, Jiaheng Zhang, Xing Ma and Yan Huang
Chem. Commun., 2018, Advance Article
DOI: 10.1039/C8CC03375G, Communication
_______________________________________________________________
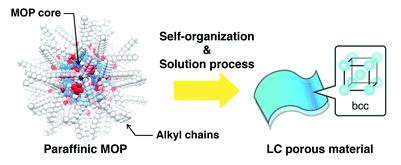
Paraffinic metal–organic polyhedrons: solution-processable porous modules exhibiting three-dimensional molecular order
Kenichiro Omoto, Nobuhiko Hosono, Mika Gochomori and Susumu Kitagawa
Chem. Commun., 2018, Advance Article
DOI: 10.1039/C8CC03705A, Communication













 Tianyu Liu obtained his Ph.D. (2017) in Physical Chemistry from University of California, Santa Cruz in the United States. He is passionate about scientific communication to introduce cutting-edge research to both the general public and scientists with diverse research expertise. He is a blog writer for Chem. Commun. and Chem. Sci. More information about him can be found at
Tianyu Liu obtained his Ph.D. (2017) in Physical Chemistry from University of California, Santa Cruz in the United States. He is passionate about scientific communication to introduce cutting-edge research to both the general public and scientists with diverse research expertise. He is a blog writer for Chem. Commun. and Chem. Sci. More information about him can be found at 



![C6CC08967D Graphical Abstract, Biological and related applications of pillar[n]arenes, Wang et al.](http://pubs.rsc.org/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C6CC08967D)
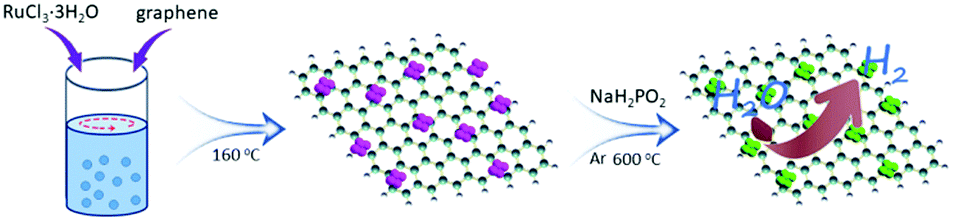
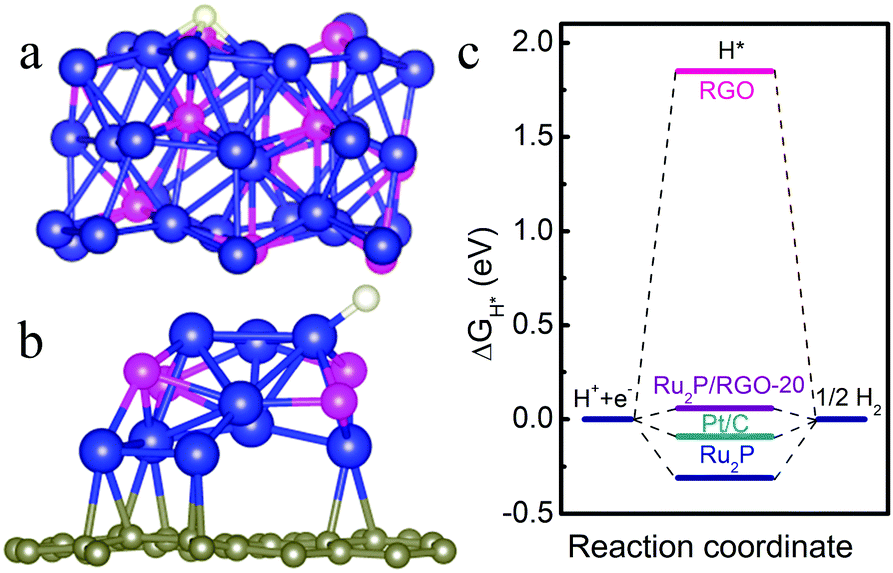
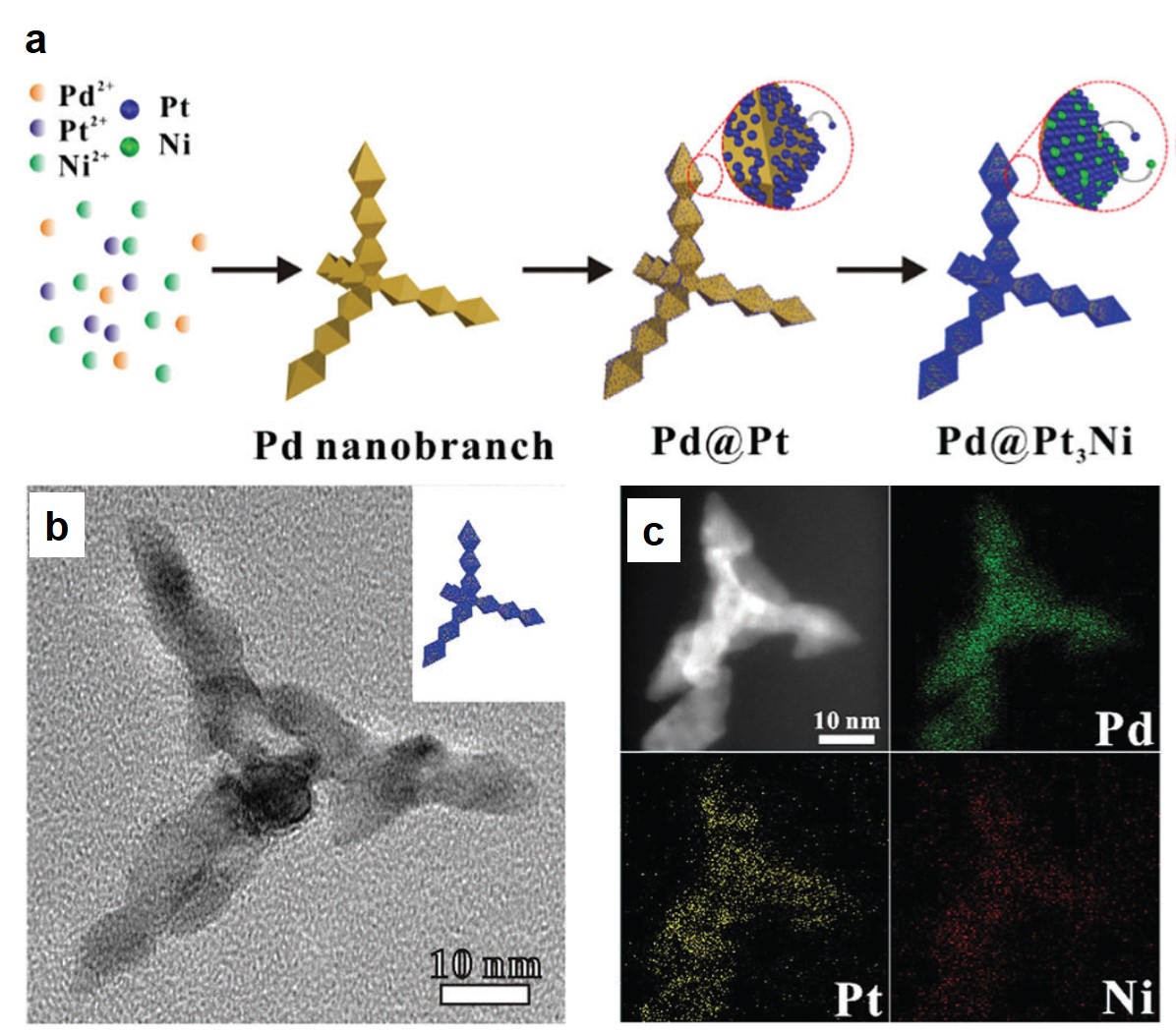
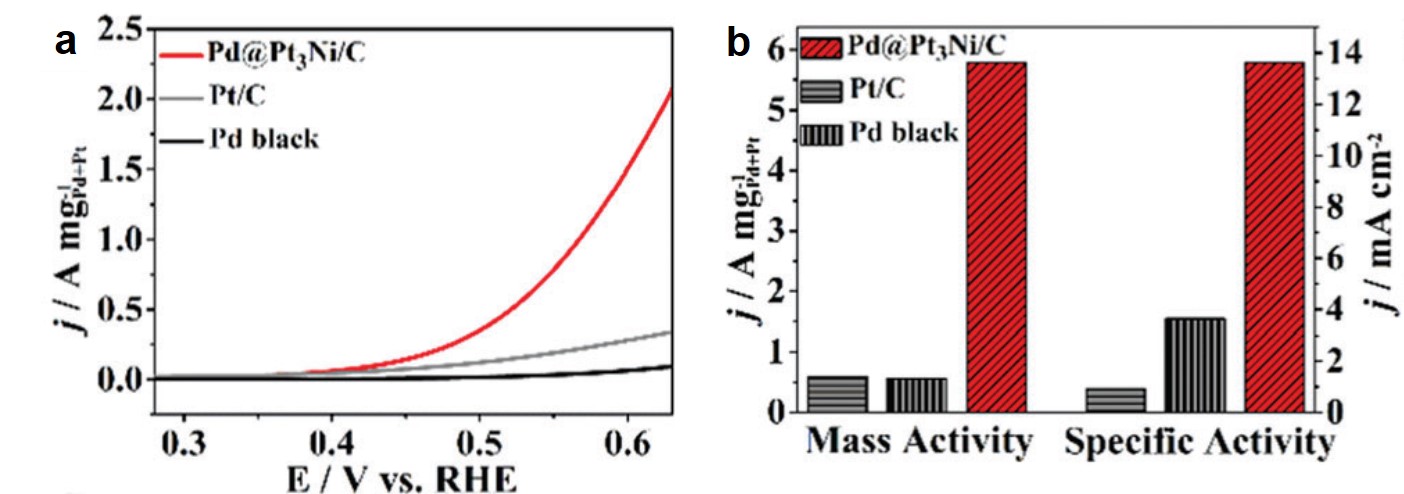
 Rational control of nano-scale metal-catalysts for biomass conversion
Rational control of nano-scale metal-catalysts for biomass conversion