Exciting news – Thanthapatra (Valentine) Bunchuay recently published his #ChemComm1st article:
Pertosylated pillar[5]arene: self-template assisted synthesis and supramolecular polymer formation. We wanted to find out about Thanthapatra’s experiences reaching a ChemComm Milestone. Find out more in our interview below.
Read about Thanthapatra and the SupraValentine group here:
What are the main areas of research in your lab and what motivated you to take this direction?
Our research in the ‘SupraValentine’ Lab at Mahidol University focuses on supramolecular and macrocyclic chemistry, which covers a broad area of interests. Learning from molecular recognition processes in nature, our lab has employed various forms of intermolecular non-covalent interactions as tools to construct supramolecular architectures. Our prime interest is the synthesis of novel macrocyclic host molecules decorated with specific functional groups to facilitate binding or encapsulation of target guest species including cations, anions, ion-pairs, and neutral molecules. These systems have been designed to self-assemble into complex nanostructures for tailored applications such as; stimuli responsive materials, smart sensors, and recovery and extraction agents. The most effective and elegant supramolecular chemistry is performed by nature and so taking inspiration from biological systems, complemented by one’s own imagination are key to my research in supramolecular chemistry. I am motivated by the idea of inspiring others to actively contribute to the exciting field of supramolecular chemistry, which is still a relatively small society in Thailand.
Can you set this article in a wider context?
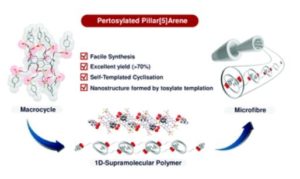
Pillar[5]arenes are a class of macrocycles having a five-fold symmetric structure constructed from electron-rich aromatic surfaces. These macrocycles can encapsulate a range of guest molecules and especially electron deficient linear alkyl moieties. Usually, these are obtained by Lewis acid catalyzed macrocyclization reactions of dialkoxy benzene monomers. However, to date the synthesis of highly decorated Pillar[5]arenes has been hindered by low yields and/or tedious synthesis. In this work, we incorporated tosylate functional groups into the monomeric unit to synthesize a pertosylated pillar[5]arene structure. The discovery of a serendipitous self-templation effect facilitated the high yielding synthesis (70%) of a perfunctionalised pillar[5]arene derivative, in contrast to the previous paradigm for decafunctionalised pillararenes of this kind. The presence of ten tosylate units not only facilitates the formation of a supramolecular polymer and nanofiber in both the solid-state and solution phase, through concerted weak hydrogen bond formation, but serves as an excellent synthetic handle in the rapid construction of highly derivatisable and multivalent nano-scafffolds. We believe that these results provide great promise for the versatility of subtle, yet powerful, unorthodox non-covalent interactions in the synthesis and functionality of supramolecular systems. Considering the rich host-guest chemistry of pillararenes we hope that our contribution to the rapid and facile access of diversifiable platforms will help further propel the inherent ‘stardom’ of pillararene based materials.
What do you hope your lab can achieve in the coming year?
My lab has been set up since August 2019. Thanks to the unfailing kindness and generosity of my previous M.Sc. supervisor, Assoc. Prof. Jonggol Tantirungrotechai I have been able to kick-start my research career. Jonggol’s sharing of space and facilities were crucial to my first independent publication. In this coming year, we hope that we will discover many secrets of nature through our systems and aim to communicate our work to the chemist community (publish more articles!). Again, thanks my students and my great colleagues especially, Prof. David Harding for your effort.
Describe your journey to becoming an independent researcher.
It’s been a long journey I would say. Since grade 10 in high school, I have received a Development and Promotion of Science and Technology Talent Project (DPST) scholarship from the Thai government to study any ‘pure science’ until Ph.D. and without hesitation chose a chemistry major. I received my B.Sc. (2011) and M.Sc.(2014) from the Faculty of Science in Mahidol University, Thailand. During that time, I was allowed to explore a broad range of chemistry from iodine mediated synthetic methodology to the post-functionalisation of MOFs for catalysis. In 2014, I had a great opportunity to join the research group of Prof. Paul D. Beer at the University of Oxford. During this time, it is no exaggeration to say my life was totally changed. It was truly an honour to be under the supervision of Paul leading with uninhibited imagination, enthusiasm and encouragement, undoubtedly influencing how I pursue science in my own research group. Apart from synthetic chemistry skills, I also learnt to be patient and deal with the dynamics of a large research group. In particular, recalling the valuable discussions with Paul and other group members helping to stimulate my own research interests. Sometimes my ideas were useful and of course some were useless, but all were useful learning experiences nonetheless. In January 2019, I secured the position of inorganic lecturer at Department of Chemistry, Faculty of Science, Mahidol University, Thailand. Starting my own research group in August 2019 with one graduate student and two undergraduate students, 10 months later we were lucky enough to publish our first work in ChemComm.
What is the best piece of advice you have ever been given?
During my time as a D.Phil student, it was certainly the most transformative and important journey of my life. I started in the Beer group with little synthetic organic experience, so my first year was a steep (but fun) learning curve. Sometimes we succeeded, many times we failed. As Paul always says “Learn to walk before you can run”, you have to keep learning, doing chemistry, and developing yourself gradually. The day that you are strong enough, you can run and jump into whatever areas that you are not familiar with. Another invaluable piece of advice from him that I really like is “Good work can be published everywhere”, however he said this when our first paper together was rejected!
Why did you choose to publish in ChemComm?
ChemComm consistently publishes many excellent works. The journal’s universally respected reputation presented the best platform to initiate my independent scientific career. The clear paper format and straightforward submission processes were also important in my decision to submit our very first publication from the ‘SupraValentine Lab’. Many thanks to the Royal Society of Chemistry (RSC) and ChemComm again for this great opportunity to support early career researchers from wherever they are in the world. This experience has encouraged me and I hope it will inspire others to continue producing good science.
Our collection of #ChemComm1st articles, including Thanthapatra’s, are available here. Don’t forget to follow us on Twitter for the latest #ChemCommMilestones news.