ChemComm Milestones celebrates emerging authors in the chemical sciences. This week, we spoke to Anna Kaczmarek who recently published her #ChemComm1st article on Ho3+–Yb3+ doped NaGdF4 nanothermometers emitting in BW-I and BW-II. Insight into the particle growth intermediate steps.

Find out more about Anna and her research below.
What are the main areas of research in your lab and what motivated you to take this direction?
My lab, the NanoSensing group, was founded in the beginning of 2020 and studies nano-sized optical sensors, specializing in nanothermometers. We have a special interest in interdisciplinary research, where the nanothermometers based on inorganic and hybrid nanomaterials can be combined with other fields such as biomedicine or reaction monitoring. We also focus part of our work on hybrid materials, such as lanthanide-grafted Covalent Organic Frameworks or lanthanide-grafted Periodic Mesoporous Organosilica, which is quite unique in the thermometry field. I have recently obtained an ERC Starting Grant on the topic of thermometry for theranostic applications, so that is currently our main theme in the research group. I have become fascinated with the topic of luminescence thermometry still during my post-doc and I am very happy I have received the chance to build a research lab at Ghent University to explore this fascinating topic.
Can you set this article in a wider context?
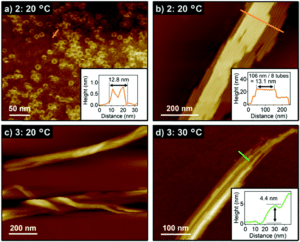
There are two interesting findings we have reported in this article – a new thermometry system based on Ho3+, Yb3+ doped 𝛽-NaGdF4 nanoparticles as well as the influence of reaction time on the 𝛽-NaGdF4 particle morphology and unique intermediate morphologies, which are formed during the transformation from 10-15 nm 𝛽-NaGdF4 spheres to 200 nm hexagonal-shaped particles.
To place the topic of the developed new thermometry system in a wider context it is important to explain that for diagnostic purposes temperature measurements in biomedicine are very important because temperature plays an essential role in biological systems. For biomedical applications accurate measurements in the so-called physiological range are crucial. It is true that detecting the temperature can be done employing more robust, and already commercially available techniques (e.g. thermocouples or infrared imaging), however optical temperature measurements at the nanoscale make it possible to revolutionize the studied resolution and reveal and research phenomena that are otherwise inaccessible to traditional thermometers. In the work we report the excellent thermal sensing capability of Ho3+, Yb3+ doped 𝛽-NaGdF4 nanoparticles, where the system is excited into the 5F5←5I8 transition of Ho3+ (640 nm) and the ratio of the 2F5/2→2F7/2 transition peak of Yb3+ and the 5I6→5I8 transition peak of Ho3+ were employed for thermometry applications. This system has previously not been explored for thermometry, however offers an excellent thermometer operating in the 1st and 2nd biological window of the human tissue. This type of system can show a high relative sensitivity in the physiological temperature regime upon measurements in water medium, without the need of shielding the Ho3+, Yb3+ doped 𝛽-NaGdF4 nanoparticle with any kind of protective silica layer despite its near infrared emission. Therefore, this is a very interesting finding for the luminescence thermometry community, where obtaining highly sensitive near infrared thermometers still remains a big challenge.
What do you hope your lab can achieve in the coming year?
I hope we can find answers and solutions to some current problems in the world of luminescence thermometry. Especially in the biomedical field there are, without doubt, still many challenges ahead of us. Also aiming for multidisciplinary materials is far from a trivial task, so we hope we will be successful in our current undertakings! Luis Carlos, an expert in lanthanide thermometry from Aveiro University, has pointed out at a congress that we need to do efforts to find real applications in the coming 10 years for the thermometers we are developing, otherwise there will be no future for this field. I take these words very seriously and will try my best to make important contributions in the field. On another level, I hope to see my research group grow and I hope I can attract new and enthusiastic researchers to come work with us. Every new person brings in a fresh perspective and a set of ideas how to solve scientific questions. I also hope to see my current students grow as researchers, and I hope that they will find joy in all the discoveries they will make during their PhDs.
Describe your journey to becoming an independent researcher.
I have always known I wanted an academic career. This might have to do with the fact that my father is an academic professor. All the biographies he brought home to me about Marie Sklodowska-Curie, whom soon became someone I idolized, definitely had a huge impact. After obtaining a Master’s degree at Adam Mickiewicz University in Poland, I decided to pursue my PhD abroad at Ghent University in Belgium in the lab of Rik Van Deun. Back then, little did I know that this was the university I would, several years later, obtain a professor title. Although I obtained a tenure track position quite young the journey was not always smooth. Funding was not always easy to acquire and there were moments in my career when I was uncertain of what the future might bring. However, I was fortunate to have people at Ghent University who believed in me and supported me when yet another funding agency rejected my post doc applications. I am very grateful for that. I also have had the opportunity to carry out several very enriching stays abroad in the labs of Francisco Romero-Salguero (Cordoba University) and Andries Meijerink (Utrecht University). They have had a huge impact on my career development and finding my own path as an independent researcher. Many colleagues in the luminescence thermometry community have also had an impact on my growth to become an independent researcher. I am very lucky to work in this supportive community. It was a bumpy road, but 2020 brought many changes. A terrible year due to the COVID-19 outbreak, but for me a very good year in many ways as I was fortunate to have been awarded the Marie Sklodowska-Curie post-doctoral fellowship, a tenure track position at Ghent University and the ERC Starting Grant, all just a few months apart. Now I have lots of work to do, and I hope to show more really exciting and relevant research in the coming years.
What is the best piece of advice you have ever been given?
I am sure there has been a huge amount of very useful advice I have received over the years working in academia and long before that. I know they have had an important impact on my development. But actually the one advice that stuck most in my head comes from a book: “When you want something with all of your heart, the universe conspires to helping you achieve it” – The Alchemist Paulo Coelho. These words kept me dreaming big and not giving up even when I was facing huge obstacles. I believed that if an academic career was what I wanted, and I worked hard enough for it, eventually it would work out. And indeed, it did. Now I am at the start of my new adventure as an independent researcher running my own lab.
Why did you choose to publish in ChemComm?
ChemComm is a renowned journal with a broad readership in chemistry. In general I am very fond of RSC journals as the review time is always fast and the process very clear and transparent.
 |
Anna M. Kaczmarek is a materials chemist studying luminescent nanothermometers and their applications in various fields such as biomedicine, high temperature industry and catalytical applications. She develops nanomaterials mostly based on lanthanide ions, however other systems based on e.g. organic dyes or silver particles have also attracted her attention.
Anna M. Kaczmarek received her master degree in chemistry from the Adam Mickiewicz University in Poznan, Poland in 2010. In 2015 she defended her PhD in Chemstry at Ghent University, Belgium. She carried out post doctoral research in 3 different groups at Ghent University and also carried out several long stays abroad at Cordoba University (Spain) and Utrecht University (The Netherlands). During this time she developed her own research line of luminescence thermometry employing inorganic and hybrid organic/inorganic nanomaterials, MOFs, COFs, and PMOs. In 2020 she obtained a permanent position at the Department of Chemistry of Ghent University (Belgium) and started the NanoSensing group, which will study nano-sized optical sensors and specialize in nanothermometry. Several leading groups in Europe and the world are already studying this important topic, however, to the best of knowledge, the NanoSensing group is the only lab in Belgium studing the emerging topic of nanothermometry. She recently obtained a prestigious ERC Starting Grant on the topic of thermometry for theranostic applications. In her work she is especially intersted in interdisciplinary research where nanothermometers based on inorganic and hybrid nanomaterials can be combined with other fields e.g. biomedicine, chemical reaction monitoring, nanoelectronics. |
Find more in ChemComm Milestones – First Independent Articles or on our Twitter, @ChemCommun.
Comments Off on ChemComm Milestones – Anna Kaczmarek