We are delighted to bring our latest ChemComm Milestones interview. This time, we would like to highlight Bogdan Barz’s #ChemComm1st article: Compact fibril-like structure of amyloid β-peptide (1–42) monomers.
Read our interview with Bogdan below.
What are the main areas of research in your lab and what motivated you to take this direction?
The focus of my group is on modeling intrinsically disordered proteins, their aggregation into highly
stable fibrils and their interaction with inhibitory peptides. One of the main aims is to quantify
protein-protein interaction and establish a direct connection to experiments. Therefore, a strong
collaboration with experimental groups is of high value. The motivation behind the research path
my group pursues is highly related to the work I did during my Ph.D., where I encountered free
energy calculation methods for the first time, but also to my later work on modeling amyloid
proteins and their self assembly. As an independent researcher I plan to combine these two topics
and make sure that my research is well anchored in experimental observations.
Can you set this article in a wider context?
The amyloid beta protein is a key protein in the onset of Alzheimer’s disease but its precise role is
not understood yet. Therefore, one should study all aspects and stages of aggregation into toxic
oligomers and fibrils in order to have a comprehensive understanding of its complex role in
Alzheimer’s disease. The monomers are the smallest species along the assembly process and
their structural diversity is a hot topic of research. Experimentally, it is difficult to study them due to
the fast aggregation into fibrils, especially for the amyloid β-protein 1-42 (Aβ42). Computationally,
there are many studies directed at monomers, generally with diverging conclusions, but there are
high hopes in the modern force fields specifically tailored for intrinsically disordered proteins. What
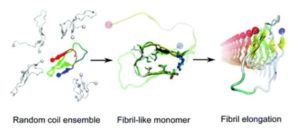
makes our study special is the finding that the structural features of the monomer model resemble
those of peptides from fibrillar structures, which is an important piece of the big puzzle. This study
explains to some degree the strong propensity of the Aβ42 monomers to aggregate into a specific
type of fibrils.
What do you hope your lab can achieve in the coming year?
Studying the structural flexibility of the Aβ42 monomer is only the first step of this project. We are
currently working on elucidating the interaction of the monomer with amyloid fibrils in a quest to
understand the relevant factors that contribute to the secondary nucleation of the amyloid beta
protein. My first Ph.D. student, Soumav Nath, is an excellent experimentalist and has already
performed many experiments planned for this project under the supervision of Prof. Alexander K.
Büll from the Technical University of Denmark. Soumav has also learned to perform molecular
dynamics simulations and is now responsible for a large part of the computational work. For the
rest of the year we will finalize the computational part of the project, corroborate the results with the
experiments and publish several related manuscripts. The funding for my group will end this fall,
but we are hoping for a more permanent status in the future.
What is the best piece of advice you have ever been given?
I always remember Dr. Nicolae Aldea’s advice that in science, as in other areas, working with
people is the most difficult task and treating co-workers with respect is what makes a great
research team.
Why did you choose to publish in ChemComm?
I find ChemComm a great journal for the diversity of topics and scientific methods used in its
published papers, but also for its fast publication process. This is my second time publishing in
ChemComm and, based on my previous experience, I can confirm that the journal has great
visibility and the published work has good chances to be cited in further studies.
Read more #ChemComm1st articles in our growing collection ChemComm Milestones – First Independent Articles. Follow us on Twitter for more #ChemCommMilestones news.