Gels are used in a number of applications, including wound dressings and fibre optics. They consist of a 3D network made up of one or more gelators held together by various non-covalent interactions (hydrogen bonding, π-π stacking, van der Waals interactions…) which serve to trap solvent molecules. Depending on the solvent and gelator used, the structure and/or viscosity of the gel can be controlled. A popular method of gel formation is through the addition of a gelator, or thickening agent, to a solution or solvent. Specifically, the development of low molecular weight gelators (LMWG) for the formation of new gels is a very active area of research. Once you can control the shape and viscosity of gels, as well as when and how they form, the possibilities for these systems are endless.
Many research groups have attempted to develop tools that allow gelation to be predicted for a given solvent, but most of these are limited to particular solvents or gelators. There is still no ‘magic bullet’ available for predicting the gelling ability of any solvent. Having said that, Matthieu Raynal and Laurent Bouteiller at UPMC University of Paris and CNRS in Paris, are one step closer towards addressing this deficiency.
Scientists can predict gel formation
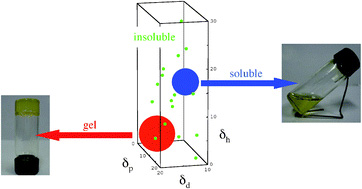
In their work, Raynal and Bouteiller applied Hansen solubility parameters (HSPs) to predict the gelling ability of LMWGs in various solvents. These parameters were initially developed to determine solubility, or to see if a given substance will dissolve in another. To reach this conclusion, three contributions must be considered: dispersive interactions, polar interactions and hydrogen bonding, and these values are then plotted on to a 3D graph, called Hansen space. The closer two molecules are to each other, the more likely they will dissolve each other. Although not without its limitations, HSPs have successfully been used by polymer chemists to select solvents for paints or coatings.
To determine if these values could be used to predict gel formation, the duo gathered information on a number of solvents and LMWG sets for which the HSPs were known. When the team plotted the information in to Hansen space, they were surprised to find for each LMWG studied that gel-forming solvents clustered together. Raynal and Bouteiller were then able to determine the so called ‘gelation sphere’ (a region of the Hansen space in which solvents forming gels were located in preference to those that form a solution or insoluble species) specific to each LMWG. They then plotted the distance between each solvent and the centre of the sphere predicting the solvents that would form gels, which were easily separated from those that formed a solution or insoluble species with a particular LMWG.
This work could prove to be a real time-saving method for predicting the gelation of a LMWG in any solvent, but first, more LMWGs need to be studied, in a wide variety of solvents. Hopefully gel scientists around the world are on the case and we will soon be able to predict gel formation of a LMWG in any solvent.
Published on behalf of web science writer, Patricia Pantos
Read the full story here:
Organogel formation rationalized by Hansen solubility parameters
Matthieu Raynal and Laurent Bouteiller
Chem. Commun., 2011, 47, 8271-8273