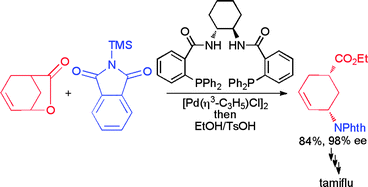
Zhenan Bao is associate professor at Stanford University, US, where she uses chemical and chemical engineering approaches to make functional nano- and microstructures with novel electronic and photonic properties. Her work has gained her international recognition, including the RSC Beilby Medal and Prize in 2009.
Zhenan Bao is associate professor at Stanford University, US, where she uses chemical and chemical engineering approaches to make functional nano- and microstructures with novel electronic and photonic properties. Her work has gained her international recognition, including the RSC Beilby Medal and Prize in 2009.
What inspired you to become a scientist?
I always had a very inquisitive nature and liked to ask ‘why?’ a lot. My parents were both scientists – one a chemist, one a physicist – and I was inspired by their careers. My father in particular instilled in me a curiosity for why things happened. He once bought me a popsicle ice lolly and asked if I thought it would sink or float in water. I said it would sink because it was solid so he threw it in the lake and said he would buy me another if we lost it. I was very surprised when it floated and, of course, wanted to learn why.
Your work focuses on organic electronics. Why did you decide to specialise in this area?
Around the time of my graduate studies at the University of Chicago, Francis Garnier reported the first printed transistor, which really inspired me. After my PhD, I joined Bell Labs (Lucent Technologies). They had already started some work in printed electronics and with my background in synthesis I thought I could immediately contribute. Following the tradition of Bell labs I was then given a lab and told to think about what I wanted to do. Organic electronics is an area with many useful applications and one where I felt I could make a real difference with my materials background.
Your work is multidisciplinary, combining chemistry, materials science, physics and various types of engineering. How do you coordinate such a diverse research group and stay on top of all the literature?
I find it very stimulating to have a group of students and post docs coming from very different backgrounds. My lab currently has nine chemists, ten chemical engineers, four electrical engineers, three physicists and several materials scientists. They all bring different expertise to the group. They learn from me and I learn from them – we are learning together. It is hard to keep on top of all the literature but if the students see some exciting work somewhere, they will let me and the whole group know so that helps a lot. I find the journal highlights very useful. They give me an idea of the current exciting work going on without having to read every issue of the journal.
What are you working on at the moment?
Lots of things! One of the areas I am most excited about is our electronic skin, where we are trying to mimic the chemical, biological and pressure sensing abilities of real skin. We also have really exciting results on transparent electrodes with carbon nanotubes, where we can achieve really good performance. We are establishing a start-up company from that work, which recently won the MIT Clean Energy Entrepreneurship prize and the Rice University business plan competition. I am very proud of the students who presented the work. In the solar cell area, we have developed new material design concepts which have started to show promising results.
How does a scientist without a business background go about setting up their own company?
I am still learning! There are other professors who have started companies so there is a lot of help and there are very helpful people who are willing to give advice. It is like doing research – when one gets stuck, one goes to the experts in the area.
Energy conversion and storage is one of the key challenges facing the modern world and something that the RSC is promoting as part of its Chemistry for Tomorrow’s World initiatives. What do you think are the main opportunities for chemists in this area?
A major part of energy research is about new materials for producing energy and also new and more energy efficient ways of making materials and chemicals. We must also take into consideration the environment – chemists can develop greener syntheses of materials and come up with materials that are more environmentally friendly. We can also develop sensors that can be used to monitor the environment. Chemistry is at the centre of all these very important global issues.
What is the best part of your job?
It is hard to rank the parts I enjoy the most. I love the interaction with students, meeting people from all over the world, being able to pursue an idea and see it become reality and also the freedom of being able to decide what to work on, who to work with. There are so many exciting possibilities and rewards for being a scientist.
You have been selected as both an outstanding young woman scientist who is expected to make a substantial impact in chemistry and one of the top 100 young innovators for this century. What is the secret to your success?
Being curious and asking lots of questions. Also being driven and willing to work hard. Being a scientist is a demanding job but there is no easy job in the world if one wants to be successful.
What is your advice to other young women thinking of embarking on a scientific career?
Women often underestimate their own ability but they must be confident and believe they can do it just as well as anybody else.
What do you do in your spare time?
I don’t have much spare time so any that I do have I spend with my two children. I try to inspire them to ask questions and think about everything they do and not take things for granted. I don’t know what they will choose to do in the future but I believe that if they develop good problem solving skills they will be successful.
If you weren’t a scientist, what would you be?
There is no other job I would like to do. Being a professor is simply the best job for me.
Comments Off on Interview: Great expectations