Ionic liquids interact with dissolved salts to give solutions that are completely different to salt solutions in traditional organic solvents or water, say UK scientists.
Ionic liquids are of great interest as green solvents but the way they solvate solutes isn’t well understood. Scientists studying their polarity have produced contradictory results – in some cases they are reported to be highly polar, in others non-polar.
Now Tom Welton and colleagues at Imperial College London say they’ve resolved this contradiction and have revealed a completely new solvent paradigm for salt solutions in ionic liquids.
In contrast to molecular solvents, where the solute cation and anion need to stay close to each other to preserve charge neutrality, ionic liquids solvate individual solute ions, explains Welton. This completely divorces the cations and anions from each other but the ionic liquid itself is capable of preserving the charge neutrality.
The polarity of ionic liquids depends on when you ask, adds Welton. Polarity measurements that record snapshots of the ionic liquid on a short timescale (such as measuring the position of the absorption maximum) ‘freeze out’ ionic movement and so the ionic liquid appears non-polar. Absorptivity measurements involve a longer timescale, allowing ion movement to dominate solvation, yielding a much higher polarity.
Read more in ‘Salts dissolved in salts‘ in Chemical Science.
Comments Off on How polar are ionic liquids?












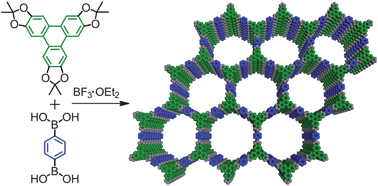 Covalent organic frameworks (COFs) are an emerging class of porous materials with potential for gas storage and organic photovoltaics. Their development has been hampered because the building blocks most commonly used to make them are poorly soluble and prone to oxidation.
Covalent organic frameworks (COFs) are an emerging class of porous materials with potential for gas storage and organic photovoltaics. Their development has been hampered because the building blocks most commonly used to make them are poorly soluble and prone to oxidation. UK scientists have worked out how ionic liquids solubilise cellulose, an important step in biomass processing.
UK scientists have worked out how ionic liquids solubilise cellulose, an important step in biomass processing.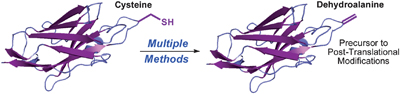 Dehydroalanine is an amino acid residue and useful precursor to a range of post-translational modifications. Several chemical and biochemical methods for incorporating dehydroalanine into peptides and proteins have been reported but each strategy has its limitations, says
Dehydroalanine is an amino acid residue and useful precursor to a range of post-translational modifications. Several chemical and biochemical methods for incorporating dehydroalanine into peptides and proteins have been reported but each strategy has its limitations, says 
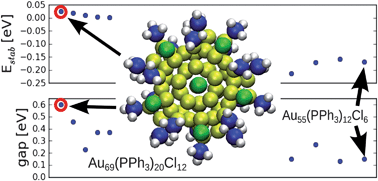 Teams from Germany, the US and Finland have re-investigated the structure of a famous gold-phosphine-halide compound.
Teams from Germany, the US and Finland have re-investigated the structure of a famous gold-phosphine-halide compound.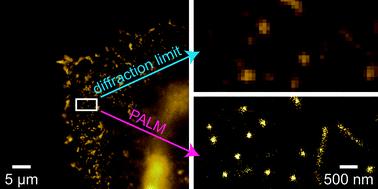 They visualised clustering of lipid molecules in nanodomains with a spatial resolution one order of magnitude better than the diffraction limit and were able to resolve the detailed structure of the domains.
They visualised clustering of lipid molecules in nanodomains with a spatial resolution one order of magnitude better than the diffraction limit and were able to resolve the detailed structure of the domains.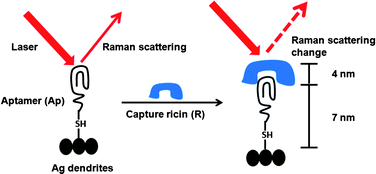 Ricin is a protein toxin naturally present in the castor bean plant (Ricinus communis); it is classified as a ‘select’ bioterror agent and was involved in a recent bioterrorism attack plot targeting US hotels and restaurants at multiple locations, as reported by the Department of Homeland Security officials in December 2010.
Ricin is a protein toxin naturally present in the castor bean plant (Ricinus communis); it is classified as a ‘select’ bioterror agent and was involved in a recent bioterrorism attack plot targeting US hotels and restaurants at multiple locations, as reported by the Department of Homeland Security officials in December 2010.